Microflow Liquid Chromatography Coupled to Multinozzle Electrospray Ionization for Improved Lipidomics Coverage of 3D Clear Cell Renal Cell Carcinoma
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
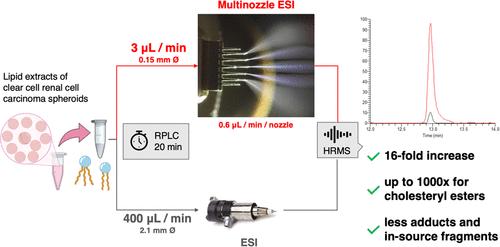
In most bioanalytical laboratories, high-resolution mass spectrometry (HRMS) systems with electrospray ionization (ESI) are hyphenated to liquid chromatography platforms. The latter typically operate under analytical flow (AF; 0.2–1 mL/min) regimes. Hence, AF/ESI-HRMS methods prioritize the detection of analytes of higher abundances or ionizability and tend to suffer from matrix effects or ion suppression. A far higher sensitivity can be obtained with electrospray at nanoflow (10–1000 nL/min) thanks to a better ionization efficiency and significant decrease in matrix effects. Both advantages are crucial to reliably accessing low-abundance compounds or weakly ionizable analytes. This work presents a microflow (μF) chromatographic setup coupled to a novel microfabricated multinozzle electrospray (mnESI) emitter with five nozzles spraying at 600 nL/min per nozzle for untargeted HRMS lipidomic profiling. With a runtime of 19 min, similar to our established analytical flow (AF/ESI) lipidomics platform, μF/mnESI produced a 16-fold median increase across 69 deuterated lipid standards. The performance of this new configuration was also evaluated in the context of the profiling of a 3D clear cell renal cell carcinoma (ccRCC) model exposed to a multidrug combination therapy. The processing of the acquired data resulted in 1270 (μF/mnESI) vs 752 (AF/ESI) MS2-annotated lipids. Among those, 762 achieved <10% variation on pooled QC samples for μF/mnESI compared to only 361 for the AF method. In addition, the measurements of ccRCC samples confirmed the improvements in ionization efficiency and adduct patterns observed with standards, enabling to annotate 79 oxidized triglycerides, 38 cholesterol esters (only five and four detected in AF/ESI, respectively), and 12 sitosterol esters, not yet reported in mammalian cell cultures.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


