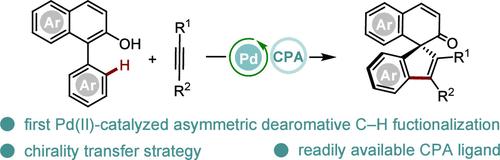
Pd(II)-Catalyzed Asymmetric C–H Functionalization/Dearomatization of Naphthols through Axial-to-Central Chirality Transfer
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
All-carbon chiral spirocycles are highly sought-after structural motifs found in various biological drugs, natural products, chiral ligands, and catalysts. However, their catalytic asymmetric synthesis remains a significant challenge due to steric hindrance and ring strain. Herein, we present the efficient synthesis of all-carbon chiral spirocycles through a Pd(II)-catalyzed asymmetric C–H functionalization/dearomatization reaction of naphthols, utilizing an axial-to-central chirality transfer strategy. This mild and versatile protocol accommodates a broad range of functionalized naphthols and alkynes, achieving good yields and enantioselectivities (up to 93% yield and 96% ee). Additionally, the practical application of this method is illustrated through the investigation of the photophysical properties of the resulting spirocycles, highlighting their potential as host materials for organic light-emitting diodes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


