Phase and Composition Engineering of Mn-Doped TiO2 for Hydrogen Peroxide Synthesis through Ion-Mediated Water Oxidation
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
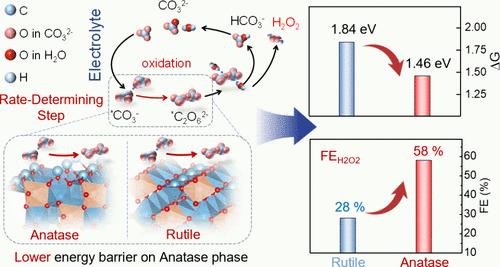
An electrochemical two-electron (2e–) water oxidation process provides a promising approach for on-site H2O2 synthesis. The design of an efficient electrocatalyst with high selectivity and productivity is crucial. In this work, manganese atoms are introduced into titanium dioxide (i.e., MnxTi1–xOy) with rutile and anatase phases for H2O2 synthesis through the two-electron water oxidation reaction. The anatase phase Mn0.08Ti0.92Oy exhibits promising activity with a low overpotential of 290 mV at 10 mA cm–2 and Faradaic efficiency of 58% for H2O2 synthesis, which is twice higher than that (∼30%) of the rutile phase counterpart. Moreover, the H2O2 production rate on the anatase Mn0.08Ti0.92Oy electrode is around 51.2 μmol min–1 cm–2, along with 600 ppm of H2O2 accumulation within an 8 min reaction. Operando infrared spectroscopic analysis combined with theoretical calculations reveals that carbonate ions in the electrolyte could effectively mediate and promote active oxygen in water oxidation on Mn0.08Ti0.92Oy for H2O2 synthesis through the peroxocarbonate intermediate pathway. Compared to the rutile phase Mn0.08Ti0.92Oy, the much lower energy barrier for the rate-determining step (oxidation of carbonate to peroxocarbonate) of the mediated two-electron water oxidation process on the anatase phase Mn0.08Ti0.92Oy is achieved for boosting the catalytic H2O2 synthesis. This work highlights an innovative phase modulation strategy and electrolyte ion assisted electrochemical process for improving the efficiency of H2O2 synthesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


