Natural Bioactive Compounds Solanesol and Chlorogenic Acid Assembled Nanomicelles for Alzheimer’s Disease Therapy
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
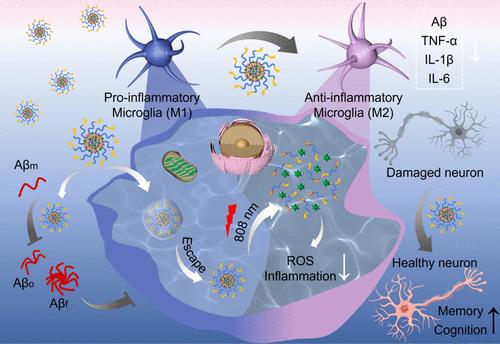
Solanesol (Sol) and chlorogenic acid (CHA) are naturally active compounds. Sol exhibits a significant free radical absorption ability and strong antioxidant activity. CHA, a typical phenolic acid, exhibits excellent anticancer, anti-inflammation, and antibacterial properties. Herein, bifunctional nanomicelles (CI@SPK) were skillfully designed to take advantage of the unique properties of Sol and CHA to treat Alzheimer’s disease (AD). Hydrophobic Sol was modified with poly(ethylene glycol) to self-assemble into stable nanomicelles (SP). CHA could be encapsulated into the hydrophobic core of these nanomicelles, which increased its bioavailability greatly. Short peptide K (CKLVFFAED) was incorporated (CI@SPK) to facilitate their crossing the blood–brain barrier. Then, CI@SPK targeted the AD lesion area, and CHA was released in greater quantities with the help of IR780 under irradiation with an 808 nm laser, resulting in synergistically scavenging reactive oxygen species (ROS) with Sol. Consequently, the nanomicelles CI@SPK demonstrated capabilities in scavenging ROS, inhibiting β-amyloid (Aβ) aggregation, and eventually modulating microglia phenotype from M1 to M2 to promote Aβ phagocytosis and clearance. In vivo studies indicated that nanomicelles CI@SPK improved the learning and cognitive impairments of APP/PS1 mice by reducing Aβ plaque and inflammation, signifying the potential value of CI@SPK in clinical application for AD treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


