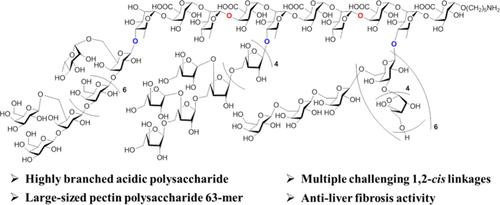
Precision Synthesis and Antiliver Fibrosis Activity of a Highly Branched Acidic 63-Mer Pectin Polysaccharide
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Natural polysaccharides possess various biological functions and have become increasingly important as drug candidates for biomedical development. However, the accessibility to multiple-branched and large-sized acidic polysaccharides with well-defined structures and the identification of related active glycan domains remain challenging. Here, we report the precision synthesis of a highly branched acidic pectin polysaccharide up to a 63-mer containing 10 different glycosidic linkages from Lycium barbarum. The synthetic strategy relies on highly stereoselective modular assembly of an orthogonally protected decasaccharide backbone, efficient synthesis of three side chain glycans by the integration of stereocontrolled one-pot chemoselective glycosylations and a hydrogen-bond-mediated aglycone delivery approach, and convergent assembly of the target polysaccharide in a branched site-specific glycosylation manner via flexible orthogonal protecting group manipulations. Structure–activity relationship studies of synthetic polysaccharide 63-mer and its short fragments (9-mer, 10-mer, 11-mer, and 33-mer) suggest that the decasaccharide as an active glycan domain exhibits better antiliver fibrosis activity.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


