Enantioselective Organocatalytic Conjugate Addition of Malonates to β,β-Disubstituted β-Trifluoromethyl Enones under High Pressure
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The first enantioselective Michael addition of malonates to acyclic β,β-disubstituted enones has been developed. Sterically hindered β-trifluoromethyl α,β-unsaturated 2-acyl thiazoles and benzothiazoles were found to be the most reactive groups of enones in the reaction catalyzed by bifunctional tertiary amine–thioureas (2–5 mol %). However, application of hyperbaric conditions (8–10 kbar) was required. The adducts containing quaternary stereogenic centers with a CF3 group were obtained in high yields (vs <1% at 1 bar) with enantiomeric excesses up to 95%.
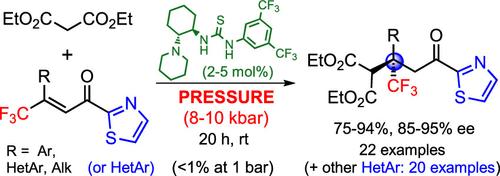
首次开发了丙二酸盐与无环 β、β-二取代烯酮的对映选择性迈克尔加成反应。研究发现,在双功能叔胺-硫脲(2-5 摩尔%)催化的反应中,受立体阻碍的β-三氟甲基α,β-不饱和 2-酰基噻唑和苯并噻唑是烯酮中反应性最强的基团。不过,需要使用高压氧条件(8-10 千巴)。含有带有 CF3 基团的四元立体中心的加合物产量很高(在 1 bar 条件下为 1%),对映体过量率高达 95%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


