Dynamic Interplay between Particle Size and Strong Metal Support Interaction in Rh/TiO2 Tuning the Selectivity of CO2 Hydrogenation
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
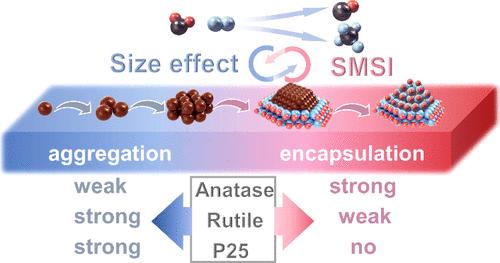
The regulation of selectivity in CO2 hydrogenation is of great importance and has been intensively studied for the utilization of CO2. The particle size and metal–support interaction are proven to be key factors influencing the selectivity of CO2 hydrogenation. However, the dynamic structure evolution during real reaction conditions and their impact on the selectivity are still poorly understood. Here in this work, we reported the crystal-phase-mediated dynamic restructuring of the Rh/TiO2 catalyst during reaction that strongly modulates the mutual interaction of dynamic size stability and strong metal–support interaction (SMSI) encapsulation of the Rh catalyst and thus the selectivity of CO2 hydrogenation toward CO/CH4. By utilizing state-of-the-art characterizations, the interplay between dynamic size distribution and SMSI of the Rh catalyst on TiO2 was clearly elucidated. The selectivity of CO2 hydrogenation was prone to the particle size in the low reaction temperature range (225–275 °C) while highly depending on SMSI at high reaction temperatures (350–400 °C). Remarkably, anatase TiO2 promotes small Rh particles and strong SMSI at the low-temperature range, rutile TiO2 facilitates large particles but high-temperature SMSI encapsulation, while the P25 phase favors large Rh particles without encapsulation. The in situ DRIFTS experiments further reveal that all Rh/TiO2 catalysts follow the hydrogenation path via *HCOO as an intermediate, where the large Rh particle size facilitates deep hydrogenation of *HCOO to CH4, whereas the TiOx encapsulation favors the *HCOO decomposition to CO due to the suppressed H2 activation. Our results provide dynamic insight for the restructuring of the active sites in the Rh/TiO2 catalyst that tunes the selectivity of CO2 hydrogenation and opens up a route for the rational design of supported metal catalysts based on their dynamic structures.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


