Ultrafast Photocatalytic Decontamination of Mustard Gas Simulant and Thermodynamic Insights by a Metal-Free Imidazoline Porous Organic Polymer
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
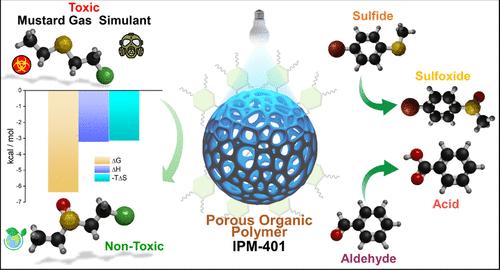
Detoxification of mustard gas through selective partial oxidation to sulfoxide is efficient yet challenging due to the hazardous nature of the overoxidized sulfone product. Metal-free imidazoline-based POPs, an emerging class of efficient photosensitizers with excellent chemical stability, incorporate both electron-rich and electron-deficient units with donor–acceptor junctions. In this study, we developed a highly photoactive protonated imidazoline-based POP, IPM-401, illustrating mild oxidizing power and enhanced ROS generation capability. Toward the detoxification of mustard gas simulant 2-chloroethyl ethyl sulfide (CEES), IPM-401 displayed excellent performance with ultrafast kinetics of t1/2 = 4.9 min in O2-saturated and t1/2 = 5.7 min under aerobic atmosphere, respectively, utilizing MeOH as the suitable solvent system. Additionally, ITC analysis revealed a favorable thermodynamic interaction (ΔG = −6.39 kcal/mol) between IPM-401 and CEES. Density functional theory calculations further validated this interaction, confirming the favorable binding of CEES to the imidazoline moiety of IPM-401. The underlying detoxification mechanism in different solvent systems is further advocated by experimental data. IPM-401 also demonstrates its versatile photocatalytic activity toward sulfide and aromatic aldehyde oxidation reactions across a broad range of substrates. Furthermore, the practical relevance of chemically stable IPM-401 was also established from its satisfactory recyclability performance up to 10 cycles.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


