Cobalt-Catalyzed Enantioselective Desymmetrization and Chemodivergent Parallel Kinetic Resolution of Unsaturated Substrates via C(sp3)–H Bond Activation
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
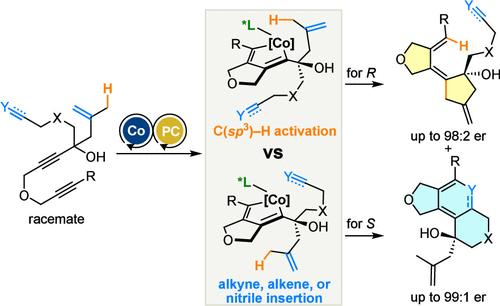
In transition-metal-mediated chemical transformations, chemoselective or chemodivergent activation of inert C–H bonds in the presence of more reactive alkene or alkyne π-bonds is a fundamental challenge. Herein, we report the Co-catalyzed cycloisomerization of unsaturated substrates bearing a 1,6-diyne moiety via enantioselective desymmetrization and parallel kinetic resolution involving the chemoselective and chemodivergent activation of a C(sp3)–H bond. The enantioselective desymmetrization of symmetrical dienediynes was demonstrated using a 1,1′-binaphthyl-based chiral phosphine ligand to afford the corresponding enantioenriched 1,3-diene products. The parallel kinetic resolution of unsymmetrical substrates such as enetriynes, dienediynes, and diynenitriles was also demonstrated, where the chemodivergent activation of a C(sp3)–H bond and an alkene, alkyne, or nitrile π-bond was achieved. The 1,3-diene products were successfully derivatized to 5–8–5 tricyclic compounds via a central-to-helical-to-central chirality transfer involving memory of chirality.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


