Defluorination of Fluorophenols by a Heme Dehaloperoxidase: Insights into the Defluorination Mechanism
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
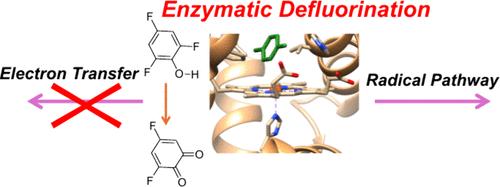
Fluorinated compounds are extensively used in industry and materials science due to their high stability and physical properties, however, they are poorly biodegradable and lead to environmental accumulation. In this work, we explore heme dehaloperoxidases for the defluorination of fluorinated aromatic compounds. Heme dehaloperoxidases are efficient enzymes that utilize H2O2 on a heme-active site to dehalogenate aromatic compounds. They usually operate by dechlorination or debromination, but recent evidence suggests defluorination is also possible; however, details of the mechanism are elusive and remain controversial. To establish the mechanism and feasibility of aromatic defluorination by dehaloperoxidase enzymes for environmental remediation and particularly the detoxification of fluorinated compounds, we performed detailed molecular dynamics (MD) and quantum mechanics studies. A complete enzyme model was created based on the dehaloperoxidase crystal structure and several fluorinated arenes were inserted. Despite the fact that the substrate binding pocket and heme active site are solvent-exposed, actually during the MD simulation, the substrates are locked inside the substrate-binding pocket through tight hydrogen bonding interactions and π-stacking interactions with amino acid residues, including several Phe amino acids and the Tyr38 and His55 side chains. We then created a large cluster model of 324 atoms of a Compound I model with 2,4,6-trifluorophenol bound and included the second-coordination sphere and studied the oxygen activation and defluorination reaction of the substrate via several possible reaction pathways. The calculations reveal a mechanism that starts with a hydrogen atom abstraction from the phenol group followed by OH rebound to the ortho- or para-positions to form 2,4,6-trifluoro-4-hydroxycyclohexadienone and 2,4,6-trifluoro-6-hydroxycyclohexadienone. These products either react inside the protein with the assistance of a proton by fluorine release or escape into the solution where the defluorination happens and the final benzoquinone products are formed. The calculations show that the hydrogen atom abstraction and OH rebound steps have small free energies of activation of <ΔG = 10 kcal mol–1, while the defluorination is rate-determining. Consequently, our studies predict that heme haloperoxidases have the potential for environmental remediation of fluorophenols from water, although a mixture of ortho- and para-activation will occur.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


