Removal of Homogeneous Broadening from 1H-Detected Multidimensional Solid-State NMR Spectra
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
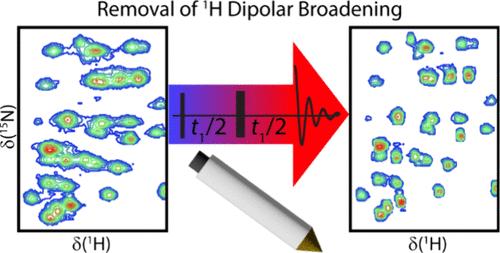
1H-detected magic-angle spinning (MAS) NMR experiments have revolutionized the NMR studies of biological and inorganic solids by providing unparalleled sensitivity and resolution. Despite these gains, homogeneous broadening, originating from the incomplete removal of homonuclear dipolar interactions under fast MAS, remains highly prevalent and limits the achievable resolution. In direct analogy to super-resolution microscopy methods, we show that resolution beyond that currently achievable by fast MAS alone can be obtained by experiment-driven deconvolution. Following the acquisition of a single 2D NMR spectrum to measure the frequency-dependent homogeneous lineshapes, any number of 1H-detected spectra can be enhanced in resolution, yielding comparable spectra as obtained with twice the MAS frequency. The versatility of this approach is demonstrated in the enhancement of single- and double-quantum homonuclear correlation spectra, in addition to heteronuclear correlation spectra acquired on a surface organometallic complex and the protein GB1.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


