Electrostatic versus Hydrogen Bonding Control of Selectivity in CO2 Reduction by Iron Porphyrins
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Multielectron, multiproton CO2 reduction selectively to C1 products is an important area of research. In nature, metalloenzymes use second-sphere interactions like hydrogen bonding and electrostatic interactions to control the rate and selectivity to these multielectron and multiproton reactions, e.g., NO2–, SO2, H+, etc. Recent developments have suggested that hydrogen bonding as well as electrostatic interactions in molecular catalysts, like iron porphyrins, results in selective 2e–/2H+ reduction of CO2, as well, to CO or HCOOH and suppresses competitive reduction of protons to H2, which occurs at similar reduction potentials. However, until date, there is no direct and systematic investigation of these two different second-sphere effects on multielectron, multiproton transformation like CO2 reduction. A series of iron porphyrins is synthesized where second-sphere hydrogen bonding and electrostatic interactions are installed in the ortho position of a mesophenyl group in an iron tetraphenyl framework. The results show that both hydrogen bonding and electrostatic interactions can facilitate the selective reduction of CO2 to CO by iron porphyrins using H2O as a proton source. In iron porphyrins with hydrogen bonding interactions, the selectivity for CO increases with an increase in H2O concentrations. However, in the iron porphyrins with electrostatic interactions, the selectivity for CO decreases, and the iron porphyrin becomes more selective for H2 evolution instead at higher H2O concentrations. Excited-state lifetime measurements and molecular dynamics simulations of porphyrins suggest that the solvation of the cationic groups in the periphery of the porphyrin by H2O leads to an increased concentration of water near the metal center, which promotes H2 evolution over CO2 reduction.
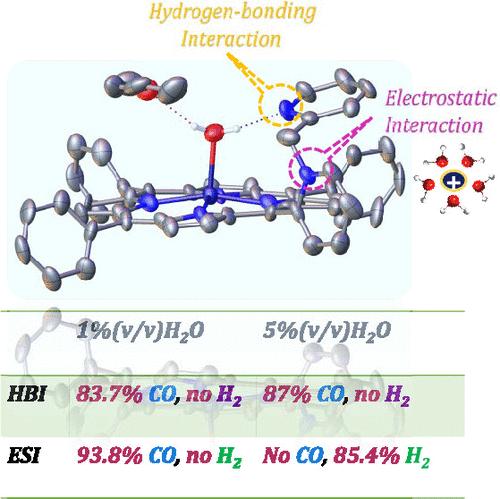
多电子、多质子 CO2 选择性还原为 C1 产物是一个重要的研究领域。在自然界中,金属酶利用氢键和静电作用等第二球相互作用来控制这些多电子和多质子反应(如 NO2-、SO2、H+ 等)的速率和选择性。最近的研究表明,分子催化剂(如铁卟啉)中的氢键和静电作用会导致 CO2 以及 CO 或 HCOOH 的选择性 2e-/2H+ 还原,并抑制质子对 H2 的竞争性还原,后者发生在相似的还原电位下。然而,迄今为止,还没有人对这两种不同的第二球效应对多电子、多质子转化(如 CO2 还原)的直接和系统研究。我们合成了一系列铁卟啉,在这些铁卟啉的四苯基框架中,介苯基的正交位置上存在第二球氢键和静电相互作用。结果表明,氢键和静电作用都能促进铁卟啉以 H2O 为质子源将 CO2 选择性地还原成 CO。在具有氢键相互作用的铁卟啉中,对 CO 的选择性随着 H2O 浓度的增加而增加。然而,在具有静电相互作用的铁卟啉中,对 CO 的选择性降低,在 H2O 浓度较高时,铁卟啉对 H2 演化的选择性反而更高。卟啉的激发态寿命测量和分子动力学模拟表明,H2O 对卟啉外围阳离子基团的溶解会导致金属中心附近的水浓度增加,从而促进 H2 演化而不是 CO2 还原。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


