Identification and Characterization of Pyrimidine Nucleoside 2′-Hydroxylase
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
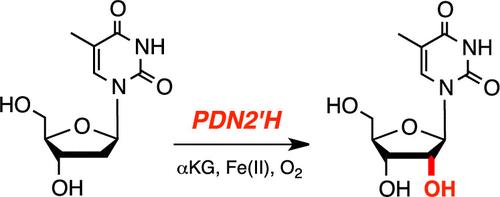
Functionalization of nucleosides at the 2′-position has become an important modification for therapeutic purposes to tailor pharmacological properties. The chemical synthesis of these molecules is challenging, and recent studies have explored bottom-up strategies with enzymes of the nucleoside salvage pathway. More than 50 years ago, a pyrimidine nucleoside 2′-hydroxylase (PDN2′H) activity had been described in fungal species extracts. However, the corresponding protein sequences were never reported and the protein characterization remained incomplete. This study describes the identification and characterization of PDN2′H from Neurospora crassa, which naturally hydroxylates thymidine at the α-2′-position as was now verified by NMR spectroscopy. Site-directed mutagenesis and biochemical assays indicated the protein to be an α-ketoglutarate-/Fe(II)-dependent dioxygenase. Furthermore, the substrate scope, phylogeny, and thermostability of NcPDN2′H were determined and its enzymatic mechanism was elucidated by resolving its X-ray protein structure cocrystallized with thymidine. NcPDN2′H is a long sought-after and important nucleoside-modifying addition to the biocatalytic portfolio.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


