Asymmetric Aza-Michael/Michael/Mannich Domino Reaction of 2-Aminochalcones and 5-Alkenyl-Thiazolones: Access to Enantioenriched 1,4-Sulfur-Bridged Piperidinone Skeletons
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we disclose a novel organocatalytic approach for the enantioselective synthesis of 1,4-sulfur-bridged piperidinone skeletons via sequential aza-Michael/Michael/Mannich domino reaction of 2-aminochalcones and 5-alkenyl-thiazolones. The one-pot approach catalyzed by a bifunctional squaramide catalyst furnishes bridged polycyclic compounds with five contiguous stereocenters (three tertiary, two heteroquaternary) in excellent yields (up to 95%) and stereochemical outcomes (up to 99% ee and up to >20:1 dr). The methodology offers outstanding control on regio- and chemoselectivity, showcasing broad substrate compatibility. Additionally, the reaction is scalable and postsynthetic transformation to a spirothiazolone-tetrahydroquinoline derivative further amplifies the synthetic utility of the methodology.
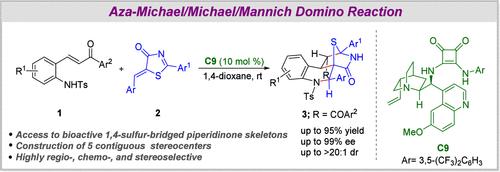
2-Aminochalcones 和 5-Alkenyl-Thiazolones 的不对称 Aza-Michael/Michael/Mannich Domino 反应:获得对映体富集的 1,4-硫杂哌啶酮骨架
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


