Metal–Organic Framework-Based Efficient Singlet Heterogeneous Photoredox Catalyst for Aerobic C–H Functionalization
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
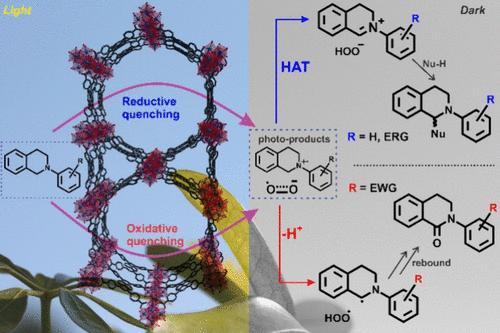
Effective light harvesting is critical in bioinspired heterogeneous photocatalysts to transport the absorbed light energy, compensating for diffusion-limited surface-only activity that is commonly achieved in solid systems constructed by simply anchoring a well-established photosensitizer (PS). While 3PS* has been widely exploited for their persistence, 1PS* defines a fresh paradigm for artificial photosystems specifically for aerobic photoredox processes where the 1O2*-mediated oxidative path must be avoided. Endowed with a large chemically accessible surface area hosting ultrafast anisotropic singlet exciton transportation, three mesoporous Zr-MOFs, PCN-222(H2), NU-1000, and SIU-100, displayed superior catalytic activities (t0.5 ∼3 h and a TOF of 106 h–1 at t0.5) toward the aerobic aza-Henry reaction of N-aryl-tetrahydroquinone compared to common 3PS* benchmarks. Furthermore, with higher excited state redox potentials, 1MOF* can be flexible in the initial photoproduct [amine•+ and O2•–] formation through an oxidative or a reductive quenching pathway expanding the scope of the amine substrates. While a slow H-atom transfer process in the dark step entails a moderate apparent quantum yield of ∼20%, a discernible rate difference was established to be defined by the driving force for the initial photoinduced electron transfer processes, which, in turn, are regulated by the electronic properties of the MOF relative to the amine substrates. Nevertheless, it is the electronic property of the amine that dictates the product identity and distribution. With detailed mechanistic studies and wider substrate scopes, this study underscores the advantageous platform for developing effective1MOF*-based heterogeneous photoredox catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


