Sodium cluster-driven safety concerns of sodium-ion batteries
IF 32.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
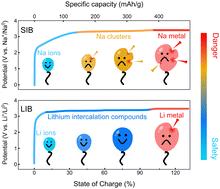
Sodium-ion batteries (SIBs) present a resource-sustainable and cost-efficient paradigm poised to overcome the limitation of relying solely on lithium-ion technologies for emerging large-scale energy storage. Yet, the path of SIBs to full commercialization is hindered by unresolved uncertainties regarding thermal safety and lingering debates over the origin of thermal runaway. Herein, through multiscale equivalent analysis from Ah-grade cells to microstructures of battery components, we probe that the difference in the chemical environment for cation storage in anodes is the mechanistic origin underlying the inferior thermal safety of SIBs compared to lithium-ion batteries (LIBs). Bearing a quasi-metallic nature, sodium clusters that form in hard carbon (HC) anodes during routine sodiation predominantly initiate cell self-exothermic reactions, significantly earlier than the decomposition of the solid–electrolyte interphase (SEI) typically observed in LIBs. Solid-state NMR measurements elucidate that clustered sodium in HC exhibits electronic properties more akin to metallic states than lithium in graphite, with even higher electron state densities at the Fermi level than bulk sodium. This heightened reactivity triggers the decomposition of linear carbonates, ultimately culminating in a thermal runaway event almost on par with scenarios involving sodium plating. Our work challenges the prevailing brief that the thermal safety insights between LIBs and SIBs are interchangeable and highlights the necessity of stabilizing deeply sodiated HC for practically safe sodium-based battery chemistries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy & Environmental Science
化学-工程:化工
CiteScore
50.50
自引率
2.20%
发文量
349
审稿时长
2.2 months
期刊介绍:
Energy & Environmental Science, a peer-reviewed scientific journal, publishes original research and review articles covering interdisciplinary topics in the (bio)chemical and (bio)physical sciences, as well as chemical engineering disciplines. Published monthly by the Royal Society of Chemistry (RSC), a not-for-profit publisher, Energy & Environmental Science is recognized as a leading journal. It boasts an impressive impact factor of 8.500 as of 2009, ranking 8th among 140 journals in the category "Chemistry, Multidisciplinary," second among 71 journals in "Energy & Fuels," second among 128 journals in "Engineering, Chemical," and first among 181 scientific journals in "Environmental Sciences."
Energy & Environmental Science publishes various types of articles, including Research Papers (original scientific work), Review Articles, Perspectives, and Minireviews (feature review-type articles of broad interest), Communications (original scientific work of an urgent nature), Opinions (personal, often speculative viewpoints or hypotheses on current topics), and Analysis Articles (in-depth examination of energy-related issues).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


