High Oxide-Ion Conduction in Rb-Containing Oxides
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
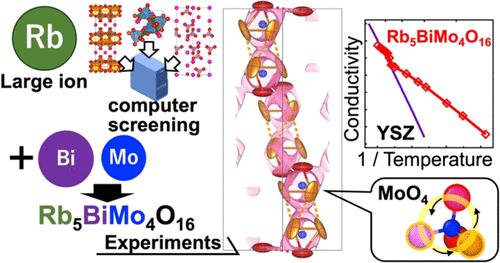
Oxide-ion conductors are essential for clean energy applications, such as solid oxide fuel cells (SOFCs). Since the Rb+ ion is the second largest available cation, some Rb-containing oxides are expected to have low activation energy for oxide-ion conductivity and high oxide-ion conductivity at low temperatures. However, Rb-containing oxide-ion conductors are very rare. Herein, we report the high oxide-ion conductivity of the palmierite-type Rb5BiMo4O16 (e.g., 2.3 mS cm–1 at 560 °C), which was discovered by a combined technique of computer screening through bond-valence-based energy calculations of 475 compositions of Rb-containing oxides, synthesis, and characterization of the structural and transport properties. Rb5BiMo4O16 exhibits a high oxide-ion conductivity of 0.14 mS cm–1 at 300 °C, which is 29 times higher than that of yttria-stabilized zirconia (YSZ) at 300 °C and comparable to the leading oxide-ion conductors with tetrahedral moieties. The high ionic conductivity below 480 °C is mainly due to the low activation energy for ionic conductivity, which can be attributed to the large free volume of Rb5BiMo4O16. The extremely large anisotropic thermal motion of oxygen atoms and the rotational motion of MoO4 tetrahedra are also responsible for the high conductivity. Rb5BiMo4O16 is stable at high temperatures under a CO2 flow, under a wet air flow, and under a wet 5% H2 in a N2 flow, and at about 21 °C in water. Rb5RMo4O16 materials (R: La, Pr, Nd, Sm, Gd, Tb, and Er) also exhibit significant conductivity. The discovery of Rb-containing oxides with high conductivity and high stability would open a new avenue for oxide-ion conductors.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


