Minocycline nanoplatform penetrates the BBB and enables the targeted treatment of Parkinson's disease with cognitive impairment
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
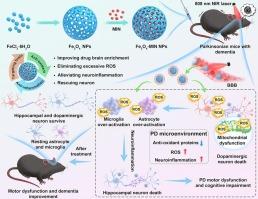
Parkinson's disease (PD)-induced motor dysfunction and cognitive impairment are becoming increasingly common due to global population aging. However, efficient treatment strategies for these conditions are still lacking. Recent studies indicated that neuroinflammation and neuronal apoptosis could greatly worsen the symptoms of PD. Therefore, anti-apoptotic and anti-inflammatory drugs could be useful in the management of PD. In the present study, minocycline (MIN)-loaded Fe3O4 nanoparticles (Fe3O4-MIN NPs) were prepared for the targeted treatment of PD. Owing to their near-infrared (NIR) irradiation-induced photothermal effects, the Fe3O4-MIN NPs could cross the blood-brain barrier (BBB), thus enhancing the delivery of Fe3O4-MIN NPs to the brain parenchyma. Subsequently, the Fe3O4-MIN NPs exerted strong anti-inflammatory effects and alleviated neuroinflammation in the brain. Furthermore, they exerted anti-oxidative effects, scavenging excessive reactive oxygen species in the brain parenchyma and thus protecting both dopaminergic and hippocampal neurons from neuroinflammation and apoptosis. Consequently, Fe3O4-MIN NPs + NIR treatment attenuated the motor dysfunction and cognitive impairment observed in PD mice. Notably, the Fe3O4-MIN NPs also showed high biocompatibility. Hence, these BBB-penetrating MIN-loaded Fe3O4 NPs demonstrate great therapeutic potential for PD accompanied by cognitive impairment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


