Regioselective intermolecular carboamination of allylamines via nucleopalladation: empowering three-component synthesis of vicinal diamines
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
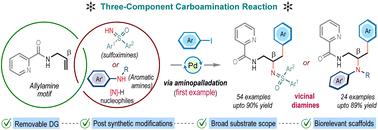
An intermolecular carboamination reaction of allyl amines under Pd(II)-catalysis is reported, expediting the synthesis of valuable vicinal diamines embedded in a functionally enriched linear carbon framework with high yields and exclusive Markovnikov selectivity. Central to our approach is the strategic use of a removable picolinamide auxiliary, which directs the regioselectivity during aminopalladation and stabilizes the crucial 5,5-palladacycle intermediate. This stabilization facilitates oxidative addition to carbon electrophiles, enabling the simultaneous incorporation of diverse aryl/styryl groups as well as important amine motifs, such as sulfoximines and anilines, across carbon–carbon double bonds. The protocol features broad substrate compatibility, tolerance to various functional groups, and scalability. The utility of this method is further demonstrated by the site-selective diversification of pharmaceutical agents. Additionally, these products serve as versatile intermediates for synthesizing heterocycles and function as effective ligands in catalytic transfer hydrogenation reactions. Notably, this work represents a rare instance of nucleopalladation-guided intermolecular carboamination of allylamines.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


