First-in-Human Study of BAT4406F, an ADCC-Enhanced Fully Humanized Anti-CD20 Monoclonal Antibody in Patients With Neuromyelitis Optica Spectrum Disorders
Abstract
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare debilitating autoimmune disease of the central nervous system (CNS). This is the first-in-human dose-escalation Phase I clinical study of BAT4406F, an antibody-dependent cell-mediated cytotoxicity (ADCC)-enhanced fully humanized anti-CD20 monoclonal antibody, in Chinese NMOSD patients.
Patients and Methods
Using a “3 + 3” design and based on the planned algorithm of dose escalation, the enrolled NMOSD patients were sequentially assigned to one of the five dose-escalation cohorts of BAT4406F with a single intravenous dose, and were then followed for a 6-month observation period. The maximum tolerated dose (MTD) and dose-limiting toxicity (DLT), safety, pharmacokinetics (PK), pharmacodynamics, and immunogenicity of BAT4406F were investigated, and the efficacy of BAT4406F in NMOSD was also preliminarily explored.
Results
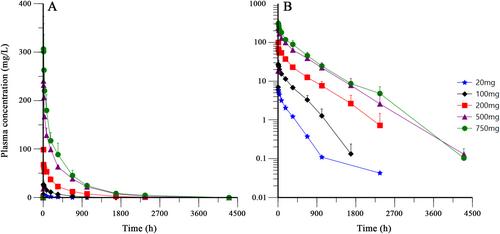
Fifteen Chinese NMOSD patients were enrolled to receive BAT4406F of escalated doses ranging from 20 to 750 mg. No subjects experienced DLT at the studied doses. BAT4406F injection exhibited favorable safety, with most of the adverse events (AE) of CTCAE Grade 1 or 2 in severity, and no Grade ≥ 3 adverse drug reactions (ADR) or serious adverse reactions occurred in any subjects. With the dose increase of BAT4406F, the maximum plasma concentration (Cmax), area under concentration-time curve from 0 to the last measurable timepoint (AUC0-t) and area under concentration-time curve from 0 to infinity (AUC0-inf) showed an increasing trend, whereas the mean clearance (CLt), terminal elimination rate (λZ), and apparent volume of distribution (Vd) decreased. The mean elimination half-life (T1/2) was ranged from 9.0–16.4 days. PK profile of BAT4406F was generally nonlinear. BAT4406F led to a rapid and significant B-cell depletion in all dose groups. Single administration of 500 mg or 750 mg maintains the CD19+ B lymphocyte count below 10/μL within the whole 6-month observation period. Three subjects were antidrug antibody (ADA) positive and all of them were neutralizing antibody (NAb)-negative. On day 99/180 postdose, several groups had decreased expanded disability status scale (EDSS) scores compared to baseline. During the observation period, NMOSD relapse occurred in two patients (13.3%) and the other 13 (86.7%) subjects remained relapse free.
Conclusion
BAT4406F was well tolerated at doses up to 750 mg and showed an expected pharmacodynamic effect of significant and long-term depletion of CD19+ B lymphocytes. It has also shown preliminary evidence of activity in NMOSD maintenance treatment, warranting further investigations.
Trial Registration
ClinicalTrials.gov identifier: NCT04146285

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


