Cascade Annulation for Synthesizing Chromenopyrrolones from o-Hydroxyphenyl Enaminones and 2-Halo-N-alkyloxyacetamides
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A cascade cyclization reaction comprising two halogenation reactions and a Michael addition was developed for the synthesis of chromeno[2,3-c]pyrrole-3-ones 4. Additionally, another cascade cyclization reaction, which involves a halogenation reaction followed by two intramolecular Michael additions, was established for the synthesis of chromeno[2,3-b]pyrrole-2-ones 5. Both types of compounds were synthesized from o-hydroxyphenyl enaminones and 2-halo-N-alkyloxyacetamides through a process that facilitated the intramolecular formation of C–C, C–O, and C–N bonds to effectively establish two fused rings in a single operation. This novel protocol is efficient, uses readily accessible starting materials, and operates under mild conditions, demonstrating tolerance for various functional groups while achieving good yields.
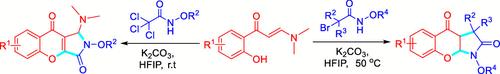
从邻羟基苯基烯酮和 2-卤代-N-烷氧基乙酰胺合成色烯吡咯酮的级联嵌合反应
为合成铬并[2,3-c]吡咯-3-酮 4,开发了一种级联环化反应,包括两个卤化反应和一个迈克尔加成反应。此外,还建立了另一种级联环化反应,包括一个卤化反应和两个分子内迈克尔加成反应,用于合成色烯并[2,3-b]吡咯-2-酮 5。这两种化合物都是由邻羟基苯基烯酮和 2-卤代-N-烷氧基乙酰胺通过分子内形成 C-C、C-O 和 C-N 键的过程合成的,从而在一次操作中有效地建立了两个融合环。这种新颖的方法效率高,使用容易获得的起始材料,操作条件温和,对各种官能团具有耐受性,同时产量高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


