Heme oxygenase 1 inhibitor discovery and formulation into nanostructured lipid carriers as potent and selective treatment against triple negative metastatic breast cancer
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Heme oxygenase-1 (HO-1) has been identified as a potential new target in anticancer therapy, being overexpressed in different tumors and crucial for cell proliferation. Advances in the development of specific HO-1 inhibitors should support the understanding of controlling HO-1 activity as antitumoral strategies, opening the path for future therapeutic applications. In the present study, small series of new HO-1 inhibitors were synthesized by joining a butylimidazolic pharmacophore together with a hydrophobic moiety spaced by a 2-oxybenzamide central linker. The most active and selective HO-1 inhibitor, VP 21–04, 2-(4-(1H-imidazol-1-yl)butoxy)-N-benzyl-5-iodobenzamide (7b) was identified. This ligand showed strong cytotoxic activity against melanoma and breast cancer cell lines. Encapsulation of VP 21–04 in nanostructured lipid carriers (NLC 21–04) was performed to exploit its therapeutic potential by passive-targeting delivery ameliorating water-solubility and toxicity. Interestingly, NLC 21–04 showed a marked antiproliferative effect in both cancer cell lines, and an improved safety profile with a wider therapeutic window when compared to the free drug. Finally, NLC 21–04 showed a marked tumor growth reduction while being safe in an in ovo tumor model, highlighting the therapeutic potential of the developed nanoparticles against triple negative metastatic breast cancer.
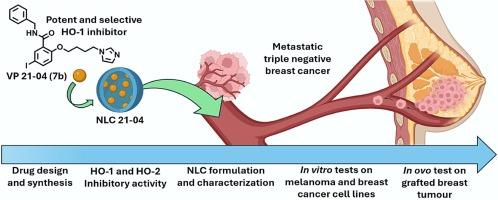
发现血红素加氧酶 1 抑制剂,并将其配制成纳米结构脂质载体,以对三阴性转移性乳腺癌进行有效的选择性治疗。
血红素加氧酶-1(HO-1)已被确定为抗癌治疗的潜在新靶点,它在不同肿瘤中过度表达,对细胞增殖至关重要。特异性 HO-1 抑制剂的开发进展应有助于理解控制 HO-1 活性的抗肿瘤策略,为未来的治疗应用开辟道路。在本研究中,通过将丁基咪唑药理结构与由 2-oxybenzamide 中心连接物间隔开的疏水分子结合在一起,合成了一系列新型 HO-1 抑制剂。结果发现了最具活性和选择性的 HO-1 抑制剂 VP 21-04,即 2-(4-(1H-咪唑-1-基)丁氧基)-N-苄基-5-碘苯甲酰胺(7b)。这种配体对黑色素瘤和乳腺癌细胞株具有很强的细胞毒活性。将 VP 21-04 包封在纳米结构脂质载体(NLC 21-04)中,通过被动靶向递送、改善水溶性和毒性来挖掘其治疗潜力。有趣的是,与游离药物相比,NLC 21-04 在两种癌细胞系中都显示出明显的抗增殖作用,而且安全性更高,治疗窗口期更宽。最后,NLC 21-04 在肿瘤模型中显示出明显的降低肿瘤生长的作用,同时保持了卵内的安全性,这突出表明了所开发的纳米粒子对三阴转移性乳腺癌的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


