Molecular polarity regulation of polybromide complexes for high-performance low-temperature zinc–bromine flow batteries
IF 32.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Frigid environments notably impair the electrochemical performance of zinc–bromine flow batteries (ZBFBs) due to polybromide solidification, restricting their widespread deployment in cold regions. Here, two independently used complexing agent cations, n-propyl-(2-hydroxyethyl)-dimethylammonium (N[1,1,3,2OH]+) and diethyl-(2-hydroxyethyl)-methylammonium (N[1,2,2,2OH]+), are proposed to enable ZBFBs to exhibit excellent performance at both low and room temperatures, through the precise regulation of the molecular polarity of polybromide complexes. Benefiting from the optimized design of the carbon number and position on the skeleton, the molecular polarity of the N[1,1,3,2OH]+- and N[1,2,2,2OH]+-polybromide complexes is appropriately reduced compared with that of choline, which is conductive to the enhancement of bromine capture capability. Interestingly, the intermolecular hydrogen bonding effect of their hydroxyl groups is not significantly enhanced, ensuring that the formed complexes maintain good fluidity at low temperatures. Thus, ZBFBs with a single complexing agent not only demonstrate an impressive average Coulombic efficiency of >95% across 1600 cycles at room temperature, but also can sustain operation with a high current density of 40 mA cm−2 for 250 cycles at −20 °C. This research significantly advances the comprehensive understanding of the mechanisms involved, thereby substantially contributing to the development of enhanced low-temperature complexing agents.
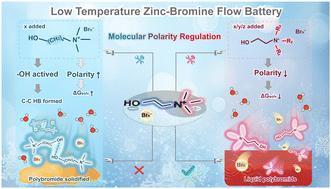
用于高性能低温锌溴液流电池的多溴化物复合物的分子极性调节
由于聚溴化物凝固,寒冷环境会明显损害锌溴液流电池(ZBFB)的电化学性能,从而限制了其在寒冷地区的广泛应用。本文提出了两种独立使用的络合剂阳离子--正丙基-(2-羟乙基)-二甲基铵(N[1,1,3,2OH]+)和二乙基-(2-羟乙基)-甲基铵(N[1,2,2,2OH]+),通过精确调节多溴化物络合物的分子极性,使 ZBFB 在低温和室温条件下都能表现出优异的性能。得益于骨架上碳数和位置的优化设计,N[1,1,3,2OH]+- 和 N[1,2,2,2OH]+- 聚溴络合物的分子极性比胆碱的分子极性适当降低,这有利于提高溴捕获能力。有趣的是,其羟基的分子间氢键效应并没有明显增强,这确保了所形成的复合物在低温下也能保持良好的流动性。因此,含有单一络合剂的 ZBFB 不仅在室温下的 1600 个循环中表现出令人印象深刻的平均库仑效率(95%),而且还能在 -20 °C 下以 40 mA cm-2 的高电流密度持续运行 250 个循环。这项研究极大地推动了对相关机理的全面了解,从而极大地促进了增强型低温络合剂的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy & Environmental Science
化学-工程:化工
CiteScore
50.50
自引率
2.20%
发文量
349
审稿时长
2.2 months
期刊介绍:
Energy & Environmental Science, a peer-reviewed scientific journal, publishes original research and review articles covering interdisciplinary topics in the (bio)chemical and (bio)physical sciences, as well as chemical engineering disciplines. Published monthly by the Royal Society of Chemistry (RSC), a not-for-profit publisher, Energy & Environmental Science is recognized as a leading journal. It boasts an impressive impact factor of 8.500 as of 2009, ranking 8th among 140 journals in the category "Chemistry, Multidisciplinary," second among 71 journals in "Energy & Fuels," second among 128 journals in "Engineering, Chemical," and first among 181 scientific journals in "Environmental Sciences."
Energy & Environmental Science publishes various types of articles, including Research Papers (original scientific work), Review Articles, Perspectives, and Minireviews (feature review-type articles of broad interest), Communications (original scientific work of an urgent nature), Opinions (personal, often speculative viewpoints or hypotheses on current topics), and Analysis Articles (in-depth examination of energy-related issues).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


