Copper-Mediated Cross-Coupling Selective for Pyroglutamate Post-Translational Modifications
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Pyroglutamate is a cyclic N-terminal post-translational modification that occurs in both proteins and peptide hormones. The prevalence and biological roles of pyroglutamate are little understood, in part due to limited tools to identify, quantify, and manipulate its pyrrolidinone structure. Selective modification of pyroglutamate residues in complex polypeptides may provide unique tools to better understand its biological roles and to allow late-stage diversification of biologically active pyroglutamate-containing sequences. This work describes a copper-catalyzed N–H cross-coupling of unprotected peptides that is selective for N-terminal pyroglutamate residues. The reaction is operationally simple under mild conditions and tolerates all canonical residues. Mechanistic studies point to a key role for a multidentate copper-binding mode of the extended polypeptide structure in delivering the observed reactivity. The reaction allows for direct labeling and identification of a pyroglutamate hormone present in porcine intestinal extracts.
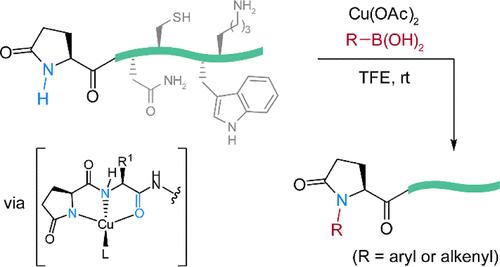
铜介导的交叉偶联选择性焦谷氨酸翻译后修饰
焦谷氨酸是一种环状 N 端翻译后修饰,在蛋白质和肽类激素中都会出现。人们对焦谷氨酸的普遍性和生物学作用知之甚少,部分原因是识别、量化和操纵其吡咯烷酮结构的工具有限。对复杂多肽中焦谷氨酸残基的选择性修饰可能提供独特的工具,以更好地了解其生物学作用,并使含焦谷氨酸的生物活性序列实现后期多样化。这项研究描述了一种铜催化的 N-H 交叉偶联反应,该反应对 N 端焦谷氨酸残基具有选择性。该反应在温和条件下操作简单,可容忍所有典型残基。机理研究表明,扩展多肽结构的多叉铜结合模式在实现所观察到的反应活性方面起着关键作用。该反应可直接标记和鉴定猪肠提取物中的焦谷氨酸激素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


