Duality application analysis of bismuth vanadate (BiVO4) as non-enzymatic glucose sensor and supercapacitor
IF 4.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Bismuth vanadate was synthesized using the hydrothermal method, with its monoclinic scheelite phase structure was confirmed by XRD. Its morphology and elemental composition confirmed by HR-SEM and XPS studies. To evaluate its efficiency in glucose sensing, cyclic voltammetry and chronoamperometry studies was used, revealing effective glucose detection with minimal interference. From the cyclic voltammetry result, it is observed that the addition of glucose resulted in a corresponding anodic peak, reflecting the oxidation of glucose. From the Chronoamperometry study, sensitivity was found to be 1.07 mA/mM cm2 for a linear range of 1 mM to 8 mM, and a limit of detection was found to be 0.12 µM. In addition, Supercapacitive performance of bismuth vanadate was also evaluated in NaOH and KOH electrolytes. It shows maximum capacitance of 423.75F/g for NaOH than KOH electrolyte (361.25F/g). Capacitance was calculated from CV and GCD measurements, and cyclic stability test showed 5000 continuous charge–discharge cycles. Thus, monoclinic scheelite bismuth vanadate demonstrates potential for both glucose sensing and supercapacitor applications.
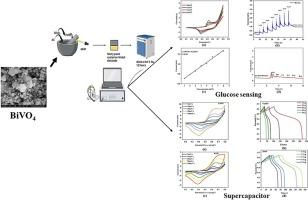
钒酸铋(BiVO4)作为非酶葡萄糖传感器和超级电容器的双重应用分析
采用水热法合成了钒酸铋,其单斜白钨矿相结构经 XRD 证实。HR-SEM 和 XPS 研究证实了其形态和元素组成。为了评估其在葡萄糖传感方面的效率,使用了循环伏安法和时变测量法进行研究,结果表明其葡萄糖检测效果显著,干扰极小。从循环伏安法的结果可以看出,加入葡萄糖会产生相应的阳极峰,反映了葡萄糖的氧化作用。根据慢性阻变研究,在 1 mM 至 8 mM 的线性范围内,灵敏度为 1.07 mA/mM cm2,检测限为 0.12 µM。此外,还评估了钒酸铋在 NaOH 和 KOH 电解质中的超级电容性能。与 KOH 电解质(361.25F/g)相比,NaOH 电解质的最大电容为 423.75F/g。电容是通过 CV 和 GCD 测量值计算得出的,循环稳定性测试显示连续充放电循环次数为 5000 次。因此,单斜白钨矿钒酸铋具有葡萄糖传感和超级电容器应用的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


