Amyloid aggregation in mixed whey proteins
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The fundamental principles behind the complexity of protein assembly, especially in mixed protein systems and crowded environments, remain elusive. This study provides molecular, structural, and viscoelastic insights into the aggregation and gelation processes in aqueous solutions of pure and mixed β-lactoglobulin and albumin whey proteins. To better understand protein aggregation in complex systems, we used a multi-technique approach that spans from molecular to macroscopic length scales. Our results show that, under low pH and heat denaturation, β-lactoglobulin tends to form ordered amyloid-type aggregates, while bovine serum albumin forms non-amyloid aggregates. In crowded environments, all protein solutions tested develop composite gel networks with distinct molecular origins. Here the ability to control the amyloid aggregate content, which has a substantial effect on the structural and viscoelastic properties of these composite gels, has been demonstrated. Gel structure and viscosity are crucial parameters to control for the food industry, as they play a key role in determining the softness and texture of food products.
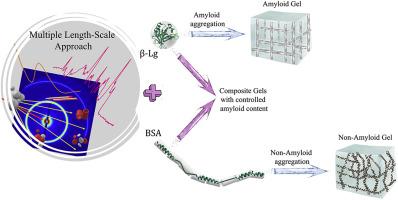
混合乳清蛋白中的淀粉样蛋白聚集
蛋白质组装复杂性背后的基本原理,尤其是在混合蛋白质体系和拥挤环境中的组装,仍然难以捉摸。本研究从分子、结构和粘弹性方面深入探讨了纯β-乳球蛋白和白蛋白乳清蛋白在水溶液中的聚集和凝胶化过程。为了更好地理解复杂系统中的蛋白质聚集,我们采用了从分子到宏观长度尺度的多技术方法。我们的研究结果表明,在低 pH 值和热变性条件下,β-乳球蛋白倾向于形成有序的淀粉样聚集体,而牛血清白蛋白则形成非淀粉样聚集体。在拥挤的环境中,所有测试的蛋白质溶液都会形成具有不同分子起源的复合凝胶网络。淀粉样蛋白聚合体的含量对这些复合凝胶的结构和粘弹性能有很大影响,而控制淀粉样蛋白聚合体含量的能力在这里得到了证实。凝胶结构和粘度是食品工业需要控制的关键参数,因为它们在决定食品的柔软度和口感方面起着关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


