Study on indole CB2 ligands based on 3D-QSAR, molecular docking and molecular dynamics simulation
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cannabinoid receptor type 2 (CB2) is a very promising therapeutic target for a variety of potential indications, such as cancer, inflammation, immune regulation and so on. However, no CB2-targeting drug has been approved to date. Therefore, it would be beneficial to explore new CB2 ligands. A 3D-QSAR study for CB2 receptor ligands were developed based on CoMFA and CoMSIA models, using 21 CB2 agonists previously reported by our laboratory. The results indicate that both CoMFA (N = 4, q2 = 0.645, r2 = 0.984) and CoMSIA (N = 6, q2 = 0.516, r2 = 0.970) have good predictive ability and stability. Contour maps of the CoMFA and CoMSIA models provide the relationship between steric effects, electrostatic effects, etc. and enhanced CB2 ligand activity. This information was used to design 1240 structurally novel compounds. The activity prediction, pharmacophore screening, molecular docking and ADME/T prediction of the designed molecules were performed in turn, and the final compound AL18 was hit. AL18 was evaluated for stability studies by molecular dynamics simulation studies. AL18 showed better stability than reference standards (GW405833, compound 2) in studied parameters such as RMSD, RMSF, Rg, etc. The binding free energy based on MM/PBSA of AL18 is −35.191 kcal mol−1, indicating strong binding interactions. AL18 can be explored as a lead compound for early research, which has not yet been experimentally explored. This study also provides a strong theoretical foundation for the subsequent design and development of indole CB2 ligands.
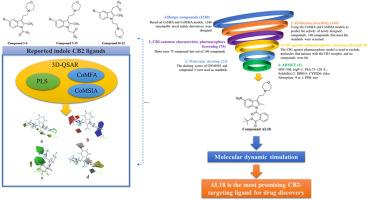
基于三维-QSAR、分子对接和分子动力学模拟的吲哚 CB2 配体研究
大麻素受体 2 型(CB2)是一个非常有前景的治疗靶点,可用于癌症、炎症、免疫调节等多种潜在适应症。然而,迄今为止还没有一种 CB2 靶向药物获得批准。因此,探索新的 CB2 配体将是有益的。基于 CoMFA 和 CoMSIA 模型,利用本实验室之前报道的 21 种 CB2 激动剂,对 CB2 受体配体进行了 3D-QSAR 研究。结果表明,CoMFA(N = 4,q2 = 0.645,r2 = 0.984)和 CoMSIA(N = 6,q2 = 0.516,r2 = 0.970)都具有良好的预测能力和稳定性。CoMFA 和 CoMSIA 模型的等高线图提供了立体效应、静电效应等与增强的 CB2 配体活性之间的关系。利用这些信息设计出了 1240 个结构新颖的化合物。对所设计的分子依次进行了活性预测、药源筛选、分子对接和 ADME/T 预测,最终确定了 AL18。通过分子动力学模拟研究对 AL18 进行了稳定性评估。在 RMSD、RMSF、Rg 等研究参数方面,AL18 比参考标准(GW405833、化合物 2)表现出更好的稳定性。根据 MM/PBSA 计算,AL18 的结合自由能为 -35.191 kcal mol-1,表明其具有很强的结合相互作用。AL18 可作为尚未进行实验探索的先导化合物进行早期研究。这项研究还为吲哚 CB2 配体的后续设计和开发提供了坚实的理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


