Fuel-Dependent Combustion Synthesis of CeCrO3 Nanomaterials: Morphological Control and Its Impact on Electrochemical Properties and Device Applications
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Perovskites exhibiting pseudocapacitance are among the leading energy storage materials as supercapacitors. The cations in perovskites significantly affect storage due to their involvement in redox reactions. CeCrO3 (CCO), a challenging-to-synthesis perovskite, has not yet been investigated for its electrochemical performance. The electrochemical properties of a material heavily depend on each step of the synthesis method. The combustion synthesis of CCO is carried out with different fuels, such as glycine (G), citric acid (C), and sucrose (S), resulting in samples CCO-G, CCO-C, and CCO-S, respectively. This makes combustion with different fuels yield products with peculiar properties and variations in morphology. Consequently, all CCO samples using different fuels exhibit noticeable differences in their electrochemical properties. A systematic investigation is conducted to understand the suitability of CCO for supercapacitor applications. Cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) techniques are performed in a 2 M KOH electrolyte. CCO-G exhibits the highest specific capacity among the samples, achieving the specific capacity of 461 C g-1 at 1 A g-1 current density. It maintains good cyclic stability of 72.98% even after 10,000 cycles at the current density of 10 A g-1. To explore its practical applicability, a hybrid supercapacitor device is fabricated with activated carbon (AC) AC@Ni-foam as the negative electrode and CCO-G@Ni-foam as the positive electrode in a 2 M KOH electrolyte. This device demonstrates a specific capacity of 204.80 C g-1 at 1 A g-1 current density. The maximum specific energy the device achieves is 36.92 Wh kg-1 at 1 A g-1 current density, and the maximal specific power is 7.91 kW kg-1 at current density of 20 A g-1. These significant values emphasize the applicability of this novel material in the field of supercapacitors, paving the way for advanced energy solutions.
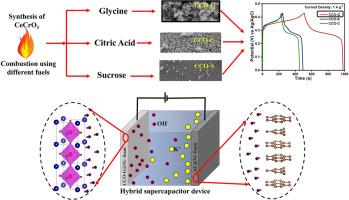
CeCrO3 纳米材料的燃料燃烧合成:形态控制及其对电化学特性和器件应用的影响
具有伪电容特性的过氧化物是作为超级电容器的主要储能材料之一。由于过氧化物中的阳离子参与氧化还原反应,因此对储能有很大影响。CeCrO3 (CCO)是一种难以合成的过氧化物,但尚未对其电化学性能进行研究。材料的电化学性能在很大程度上取决于合成方法的每个步骤。CCO 的燃烧合成采用不同的燃料,如甘氨酸(G)、柠檬酸(C)和蔗糖(S),分别得到 CCO-G、CCO-C 和 CCO-S 样品。这使得不同燃料燃烧产生的产物具有特殊的性质和形态变化。因此,使用不同燃料的所有 CCO 样品在电化学特性上都表现出明显的差异。为了解 CCO 在超级电容器应用中的适用性,我们进行了一项系统调查。在 2 M KOH 电解液中进行了循环伏安法 (CV) 和电静态充放电 (GCD) 技术研究。在所有样品中,CCO-G 的比容量最高,在电流密度为 1 A g-1 时,比容量达到 461 C g-1。即使在 10 A g-1 的电流密度下循环 10,000 次,它仍能保持 72.98% 的良好循环稳定性。为了探索其实际应用性,我们在 2 M KOH 电解液中以活性炭 (AC) AC@Ni 泡沫为负极,以 CCO-G@Ni 泡沫为正极,制作了一种混合超级电容器装置。该装置在 1 A g-1 电流密度下的比容量为 204.80 C g-1。在电流密度为 1 A g-1 时,该装置的最大比能量为 36.92 Wh kg-1;在电流密度为 20 A g-1 时,最大比功率为 7.91 kW kg-1。这些重要数值强调了这种新型材料在超级电容器领域的适用性,为先进的能源解决方案铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


