Enantioselective Synthesis of vic-Aminoalcohol Derivatives by Nickel-Catalyzed Reductive Coupling of Aldehydes with Protected Amino-pentadienoates
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A VAPOL-derived phosphoramidite ligand is uniquely effective at reverting the regiochemical course of nickel-catalyzed reactions of aldehydes with carbamate-protected 5-amino-2,4-pentadienoates as “push/pull” dienes; the ensuing carbonyl α-amino-homoallylation reaction affords anti-configured vic-aminoalcohol derivatives in good yields with high optical purity. The reductive coupling is conveniently performed with a bench-stable Ni(0) precatalyst and Et3B as the promoter.
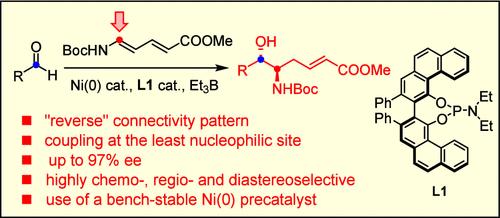
通过镍催化醛与受保护氨基戊二烯酸酯的还原偶联,对映选择性合成vic-氨基乙醇衍生物
在镍催化的醛与氨基甲酸酯保护的 5-氨基-2,4-戊二烯酸作为 "推/拉 "二烯的反应中,一种源自 VAPOL 的磷酰胺配体能独特有效地逆转反应的区域化学反应过程;随后的羰基 α-氨基-高烯丙基化反应能以良好的收率和高光学纯度生成反构型的沧胺醇衍生物。该还原偶联反应使用台式稳定的 Ni(0) 前催化剂和 Et3B 作为促进剂,非常方便。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


