Synthesis of Multisubstituted Cyclopentadiene Derivatives from 3,3-Disubstituted Cyclopropenes and Internal Alkynes Catalyzed by Low-Valent Niobium Complexes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A low-valent niobium species generated from NbCl5 and 1-methyl-3,6-bis(trimethylsilyl)-1,4-cyclohexadiene (Si-Me-CHD) in combination with PPh3 catalyzed a [2+2+1]-cycloaddition reaction of 3,3-disubstituted cyclopropenes and 2 equiv of diaryl/dialkylalkynes, leading to isomeric mixtures of multisubstituted cyclopentadienes 3–5. The initial catalyst activation process was a one-electron reduction of NbCl5 with Si-Me-CHD to provide [NbCl3(μ-Cl) (L)]2 (L = PMe2Ph (6), L = PPh3 (7)) in the presence of phosphine ligands. An NMR spectroscopic time course experiment using complex 7 as the catalyst revealed an induction period for the product formation, corresponding to an additional one-electron reduction of 7 by the substrates to give catalytically active η2-alkyne complexes of NbCl3. A combined computational and experimental study clarified the mechanism of this unprecedented [2+2+1]-cyclopentadiene synthesis; a rate-determining 1,2-insertion of cyclopropene into η2-alkyne niobium species to form cyclopropane-fused metallacyclopentene followed by ring-opening β-C elimination provides a dienylalkylidene intermediate prior to incorporation of the second alkyne through carbene/alkyne metathesis. We also demonstrated the synthetic utility of the multisubstituted cyclopentadienes as the cyclopentadienyl ligands by derivatizing to the corresponding lithium cyclopentadienide, which is applicable for the synthesis of ferrocene 10.
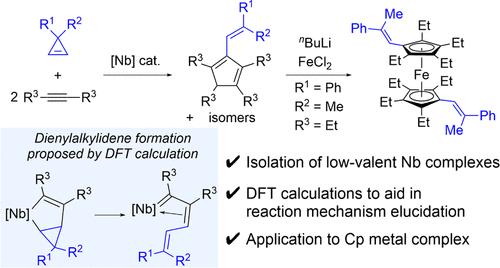
在低价铌络合物催化下,从 3,3-二取代环丙烯和内部炔合成多取代环戊二烯衍生物
由 NbCl5 和 1-甲基-3,6-双(三甲基硅基)-1,4-环己二烯(Si-Me-CHD)与 PPh3 结合生成的低价铌物种催化了 3,3-二取代环丙烯和 2 等量二芳基/二烷基炔的[2+2+1]-环加成反应,生成了多取代环戊二烯 3-5 的异构混合物。最初的催化剂活化过程是 NbCl5 与 Si-Me-CHD 的一电子还原,在膦配体存在下生成 [NbCl3(μ-Cl) (L)]2(L = PMe2Ph (6),L = PPh3 (7))。以络合物 7 为催化剂进行的核磁共振光谱时程实验显示,生成物的形成有一个诱导期,相当于底物对 7 进行额外的单电子还原,生成具有催化活性的 NbCl3 η2-炔络合物。计算和实验相结合的研究阐明了这一前所未有的 [2+2+1]- 环戊二烯合成的机理;在通过碳烯/炔烃偏聚作用加入第二个炔烃之前,环丙烯与 η2-炔烃铌复合物发生了决定速率的 1,2-插入反应,形成环丙烷融合的金属环戊烯,随后发生开环 β-C 消去反应,产生了一个亚烷基二烯中间体。我们还通过衍生成相应的环戊二烯锂,证明了多取代环戊二烯作为环戊二烯配体的合成用途,这种配体可用于合成二茂铁 10。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


