Shapeshifting Ligands Mask Lewis Acidity of Dicationic Palladium(II)
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Supporting ligands limit the degree of electrophilic activation for any substrate because they also reduce the Lewis acidity of the transition metal ion. Here, we temporarily mask the Lewis acidity of dicationic Pd(II) by using “shapeshifting” bidentate pyrimidine/olefin ligands L1 and L2. These ligands delocalize/relocalize charge via reversible C–N bond formation. So, although ligated dicationic Pd compounds [1]2+ and [2]2+ appear charge separated (distributed across Pd and ligand), they react comparably to a solvated Pd(II) dication. Despite reacting like strong Lewis acids, the complexes are tolerant of polar functional groups (Lewis bases that often inhibit electrophilic catalysis). We propose that this property originates from the installation of a more nucleophilic (charge separated) state. This case study suggests that catalysts featuring reversible dynamics can be advantageous relative to their structurally static counterparts.
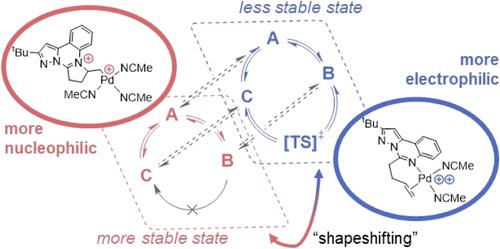
变形配体掩盖二价钯(II)的路易斯酸性
支撑配体限制了任何底物的亲电活化程度,因为它们也降低了过渡金属离子的路易斯酸度。在这里,我们通过使用 "变形 "双齿嘧啶/烯烃配体 L1 和 L2,暂时掩盖了二价钯(II)的路易斯酸性。这些配体通过可逆的 C-N 键形成来分散/重新定位电荷。因此,虽然配位的二阳离子钯化合物 [1]2+ 和 [2]2+ 看起来电荷分离(分布在钯和配体上),但它们的反应与溶解的二阳离子钯相当。尽管反应类似于强路易斯酸,但这些配合物对极性官能团(通常会抑制亲电催化作用的路易斯碱)具有耐受性。我们认为这一特性源于亲核(电荷分离)状态的形成。这一案例研究表明,具有可逆动力学特征的催化剂相对于结构上静态的催化剂更有优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


