Copper-Catalyzed Three-Component Tandem Cyclization for One-Pot Synthesis of Indole-Benzofuran Bis-Heterocycles
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A one-pot, three-component synthesis of indole-benzofuran bis-heterocycles from terminal alkynes, salicylaldehydes, and indoles has been developed via copper-catalyzed tandem annulation. This catalytic system utilizes readily available starting materials, enabling predictable synthesis of indole-benzofuran bis-heterocycles with broad substrate versatility, excellent regiocontrol, and gram-scale amenability. The reaction proceeds via a sequential pathway involving A3 coupling, 1,4-conjugate addition, and 5-exo-dig cyclization.
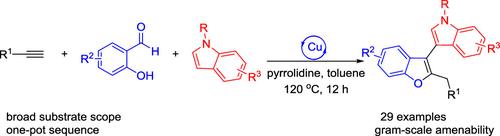
铜催化三组分串联环化技术用于吲哚-苯并呋喃双杂环的一锅合成
通过铜催化的串联环化反应,开发出了一种以端炔烃、水杨醛和吲哚为原料的吲哚-苯并呋喃双杂环的单锅三组分合成方法。该催化系统利用容易获得的起始材料,能够以可预测的方式合成吲哚-苯并呋喃双杂环,具有广泛的底物通用性、出色的调节控制和克级适配性。反应通过 A3 偶联、1,4-共轭物加成和 5-外-二元环化的顺序途径进行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


