Excipient Induced Unusual Phase Separation in Bovine Serum Albumin Solution: An Explicit Role Played by Ion-Hydration
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report an instantaneous room-temperature phase separation of 1 mM bovine serum albumin solution in the presence of (20% acetic acid+0.2 M NaCl), a routinely used food preservative; an opaque liquid-like phase (L) coexists in equilibrium with a granular gel like phase (G). Interestingly, neither 20% acetic acid nor 0.2 M NaCl individually induces such a phase separation. Field emission scanning electron microscopy (FESEM) and high-resolution transmission electron microscopy (HRTEM) imaging show aggregated proteins to be dispersed in the upper phase, while the lower phase is composed of cross-linked fibrils (hydrogels). Mid-IR FTIR, Raman scattering, and circular dichroism (CD) experiments reveal a significant increase in the β-sheet content in BSA, which confirms the formation of amyloids in the presence of the excipient. Both L and G phases undergo distinct visual and microscopic changes upon incubation at 25 and 80 °C. It is evident that the added salt plays a pivotal role in bringing about this otherwise unique phase behavior. We divulge the explicit role of the ion associated hydration using THz-FTIR measurements in the 1.5–16.7 THz (50–550 cm–1) frequency window. Systematic alteration in the ion-induced THz-active mode of water envisions the key role of ions in shaping the various phases. Our study depicts an intriguing observation of severe amyloidosis of BSA upon the addition of a food preservative, even at room temperature, which is expected to add new insight into amyloid research. Considering the increasing number of individuals suffering from several neurodegenerative disorders (Alzheimer’s, Parkinson’s, type-2 diabetes, obesity, cancer, etc.), this study leads a caution toward critically revisiting the usage of known food preservatives.
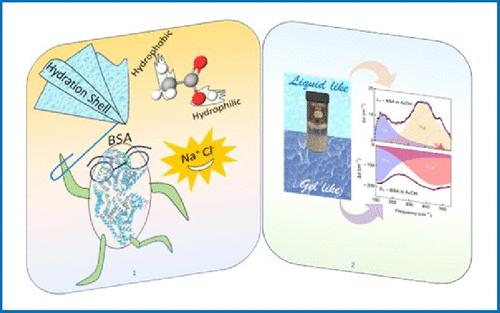
牛血清白蛋白溶液中辅料诱发的异常相分离:离子水合的明显作用
我们报告了 1 mM 牛血清白蛋白溶液在常规食品防腐剂(20% 乙酸+0.2 M NaCl)存在下的瞬时室温相分离现象;不透明的液态相(L)与颗粒状的凝胶体相(G)共存于平衡状态。有趣的是,20% 的醋酸和 0.2 M 的氯化钠都不能单独诱导这种相分离。场发射扫描电子显微镜(FESEM)和高分辨率透射电子显微镜(HRTEM)成像显示,聚集的蛋白质分散在上相中,而下相则由交联的纤维(水凝胶)组成。中红外傅立叶变换红外光谱(FTIR)、拉曼散射和圆二色性(CD)实验显示,BSA 中的β片含量显著增加,这证实了在辅料存在的情况下淀粉样蛋白的形成。L 相和 G 相在 25℃和 80℃下孵育时都会发生明显的视觉和显微变化。很明显,添加的盐在形成这种独特的相行为中起到了关键作用。我们利用 1.5-16.7 THz(50-550 cm-1)频率窗口的 THz-FTIR 测量,揭示了离子相关水合的明确作用。离子诱导的水的太赫兹活性模式的系统性变化预示着离子在塑造各种物相中的关键作用。我们的研究描绘了一个有趣的观察结果,即在添加食品防腐剂后,即使在室温下,BSA 也会发生严重的淀粉样变性,这有望为淀粉样蛋白研究增添新的见解。考虑到越来越多的人罹患多种神经退行性疾病(阿尔茨海默氏症、帕金森氏症、2 型糖尿病、肥胖症、癌症等),本研究提醒人们要认真重新审视已知食品防腐剂的使用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


