Exploring Cationic Substitutions in the Solid Electrolyte NaAlCl4 with Density Functional Theory
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
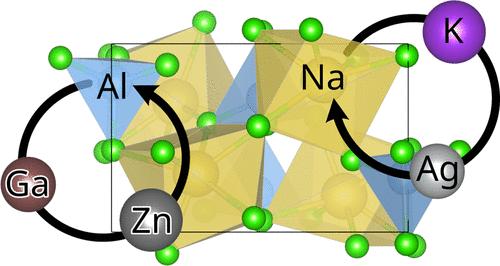
NaAlCl4 is an established solid electrolyte in high-temperature Na-based battery systems, but its ionic conductivity is not sufficiently high for room-temperature applications. We employ density functional theory and thermodynamic corrections to evaluate the efficacy of various elements for substitution, utilizing on-the-fly machine-learned potentials to accelerate the required phonon calculations by 1 order of magnitude at a minor error of −0.7 ± 1.0 meV/atom. All investigated isovalent substitutions are favorable within 4 meV/atom, with potassium and silver as substitutes for sodium and gallium as a substitute for aluminum. The most promising aliovalent substitution was identified for Zn on the tieline between NaAlCl4 and Na2ZnCl4. The structure of latter, with aluminum ions replacing zinc, yields a structure with separate layers for the differently charged cations and vacancies for potential Na conduction. Our investigation may pave the way for more reliable discovery of new Na conductors by inclusion of thermodynamic properties.
用密度泛函理论探索固体电解质 NaAlCl4 中的阳离子置换
NaAlCl4 是高温钠基电池系统中一种成熟的固体电解质,但其离子电导率在室温应用中不够高。我们采用密度泛函理论和热力学修正来评估各种元素的替代功效,利用即时机器学习的电势将所需的声子计算速度提高了 1 个数量级,微小误差为 -0.7 ± 1.0 meV/原子。所研究的所有异价置换在 4 meV/原子内都是有利的,钾和银可替代钠,镓可替代铝。在 NaAlCl4 和 Na2ZnCl4 之间的铁线上发现了最有希望的锌的别价取代。后者的结构中,铝离子取代了锌,从而产生了一种结构,其中不同电荷的阳离子有不同的层,而空位则可用于潜在的 Na 传导。我们的研究可通过纳入热力学性质,为更可靠地发现新的 Na 导体铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


