Dipeptidic Proline Amide–Isothiouronium Salt Organocatalysts for Enantiodivergent Conjugate Addition Reactions Between Aldehydes and Nitroolefins
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We rationally designed and successfully developed novel dipeptidic proline amide–isothiouronium catalysts for asymmetric conjugate addition reactions between various aldehydes and nitroolefins, which generated 1,4-addition products with up to 95% yields, 92:8 syn-diastereoselectivity, and 96% enantiomeric excess. The catalysts, which were prepared via simple methylation of the corresponding thiourea, can provide the desired enantiomeric syn-1,4-adducts by exchanging the configuration of the N-terminal proline moiety.
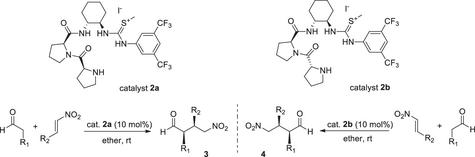
二肽脯氨酸酰胺-异硫脲盐有机催化剂用于醛与硝基烯烃的对映异构共轭加成反应
我们合理设计并成功开发了新型二肽脯氨酸酰胺-异硫脲催化剂,用于各种醛和硝基烯烃之间的不对称共轭加成反应,生成的 1,4 加成产物收率高达 95%,同步-非对映选择性为 92:8,对映体过量率为 96%。这些催化剂是通过相应硫脲的简单甲基化制备的,可以通过交换 N 端脯氨酸分子的构型来提供所需的对映体合成 1,4 加合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


