Substrate Directed Highly Diastereoselective Synthesis of Eliglustat: A Drug for Gaucher Disease
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A highly diastereoselective total synthesis of Eliglustat has been accomplished starting from a readily available D-serine. This novel route involves a four-step telescoped process to afford the keto intermediate (4) in 74% overall yield. The diastereoselective reduction of 4 using sodium borohydride provides the alcohol, a key intermediate 5, with excellent selectivity (>99 dr) and yield (95%). The total synthesis of Eliglustat from D-serine has been accomplished in ten steps with an overall yield of 21%. This process not only gives a desirable yield but also avoids the use of hazardous conditions.
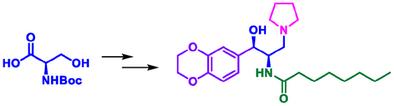
以底物为导向高非对映选择性合成艾格司他:治疗戈谢病的药物
我们从易于获得的 D-丝氨酸开始,完成了一种高非对映选择性的艾格司他全合成方法。这条新颖的路线包括一个四步伸缩过程,以 74% 的总收率获得酮中间体 (4)。使用硼氢化钠对 4 进行非对映选择性还原,可得到醇,即关键的中间体 5,具有极佳的选择性(99 dr)和收率(95%)。以 D-丝氨酸为原料,通过十个步骤完成了 Eliglustat 的全部合成,总收率为 21%。该工艺不仅产量理想,而且避免了使用危险条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


