Comparative study of gut microbiota and metabolite variations between severe and mild acute pancreatitis patients at different stages
IF 3.3
3区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Acute pancreatitis (AP) is influenced by interactions between gut microbiota and metabolic products, though the mechanisms remain unclear. This study investigates variations in gut microbiota and metabolites between severe (SAP) and mild acute pancreatitis (MAP) patients to assess their impact on disease progression. Using a cross-sectional cohort design, gut microbiota and metabolite profiles were compared in SAP and MAP patients over two weeks post-diagnosis. 16S rRNA gene sequencing and metabolomic analyses, including KEGG pathway assessments and Spearman correlation, were employed, along with Mendelian Randomization (MR) to assess the influence of specific microbiota on AP. Results showed that SAP patients had significantly reduced gut microbiota diversity, which further declined in the second week. This was marked by increases in pathogenic bacteria like Stenotrophomonas and Enterobacter and decreases in beneficial bacteria such as Blautia. Key changes included a rise in Proteobacteria and a decline in Ruminococcaceae, Enterococcus, and Faecalicatena. Metabolic shifts included lipid metabolite upregulation and antioxidant downregulation. Correlation analysis linked Stenotrophomonas to short-chain fatty acid and amino acid metabolism, highlighting its role in disease progression. MR analysis confirmed negative causal relationships between Enterococcus B, Faecalicatena torques, and AP, suggesting protective effects. Variations in Blautia species indicated differing influences on AP. This study underscores the critical role of gut microbiota and metabolites in AP progression and suggests the need for further research to confirm these findings and explore targeted therapeutic interventions.
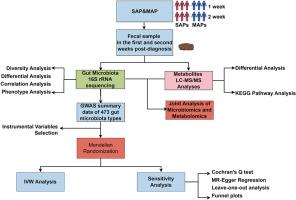
不同阶段重度和轻度急性胰腺炎患者肠道微生物群和代谢物变化的比较研究
急性胰腺炎(AP)受肠道微生物群和代谢产物之间相互作用的影响,但其机制仍不清楚。本研究调查了重症(SAP)和轻症急性胰腺炎(MAP)患者肠道微生物群和代谢产物的变化,以评估它们对疾病进展的影响。采用横断面队列设计,比较了 SAP 和 MAP 患者在确诊后两周内的肠道微生物群和代谢物谱。采用了 16S rRNA 基因测序和代谢组学分析,包括 KEGG 通路评估和斯皮尔曼相关性分析,以及孟德尔随机化(MR)来评估特定微生物群对 AP 的影响。结果显示,SAP 患者的肠道微生物群多样性明显降低,在第二周进一步下降。这主要表现为致病菌(如血吸虫病单胞菌和肠杆菌)的增加和有益菌(如布劳氏菌)的减少。主要变化包括变形菌增加,反刍球菌、肠球菌和粪肠球菌减少。代谢变化包括脂质代谢物上调和抗氧化剂下调。相关性分析将血吸单胞菌与短链脂肪酸和氨基酸代谢联系起来,突出了其在疾病进展中的作用。磁共振分析证实了肠球菌 B、Faecalicatena torques 和 AP 之间的负因果关系,表明它们具有保护作用。Blautia 种类的变化表明对 AP 有不同的影响。这项研究强调了肠道微生物群和代谢物在 AP 进展过程中的关键作用,并建议有必要开展进一步研究,以证实这些发现并探索有针对性的治疗干预措施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microbial pathogenesis
医学-免疫学
CiteScore
7.40
自引率
2.60%
发文量
472
审稿时长
56 days
期刊介绍:
Microbial Pathogenesis publishes original contributions and reviews about the molecular and cellular mechanisms of infectious diseases. It covers microbiology, host-pathogen interaction and immunology related to infectious agents, including bacteria, fungi, viruses and protozoa. It also accepts papers in the field of clinical microbiology, with the exception of case reports.
Research Areas Include:
-Pathogenesis
-Virulence factors
-Host susceptibility or resistance
-Immune mechanisms
-Identification, cloning and sequencing of relevant genes
-Genetic studies
-Viruses, prokaryotic organisms and protozoa
-Microbiota
-Systems biology related to infectious diseases
-Targets for vaccine design (pre-clinical studies)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


