Baicalin attenuates corticosterone-induced hippocampal neuronal injury by activating mitophagy in an AMPK-dependent manner
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Defective mitophagy is closely related to the neuronal dysfunction and major depressive disorder (MDD). Our previous study found that baicalin could enhance nip-like protein (NIX)-mediated mitophagy and exhibit antidepressant effects, and predicted that AMPK may be the pharmacological target of baicalin. However, validated experiments are lacking. In the present study, we first demonstrated the effect of baicalin on hippocampal NIX-mediated mitophagy in CORT-induced depressive mice. Secondly, we transfected siRNA to knockdown AMPK, PGC-1α, and NIX respectively in HT22 cells, and detected the effects of baicalin on mitochondrial function, AMPK/PGC-1α/NIX pathway protein expression and mitophagy levels. Finally, AAV-shAMPKα was injected into hippocampal CA3 to knockdown AMPK in mice to validate the antidepressant effects of baicalin in vivo. The results showed that CORT induced depressive-like behaviors, accompanied with neuronal damage, mitochondrial injury, and inhibited mitophagy in the hippocampus, which were prevented by baicalin (20 mg/kg) treatment. In HT22 cells, baicalin remarkably ameliorated mitochondrial dysfunction and mitophagy disturbance induced by CORT, and these protective effects of baicalin were blocked by knockdown of AMPK, PGC-1α and NIX. Moreover, the beneficial effects of baicalin on depressive-like behaviors and NIX-mediated mitophagy were suppressed by knockdown of AMPKα in mice. Our present results further demonstrated that baicalin promotes NIX-mediated mitophagy and improves depression in an AMPK-dependent manner.
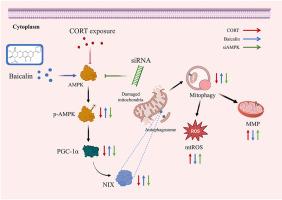
黄芩苷以AMPK依赖性方式激活有丝分裂,从而减轻皮质酮诱导的海马神经元损伤
有丝分裂缺陷与神经元功能障碍和重度抑郁症(MDD)密切相关。我们之前的研究发现,黄芩苷能增强nip样蛋白(NIX)介导的有丝分裂并表现出抗抑郁作用,并预测AMPK可能是黄芩苷的药理靶点。然而,目前还缺乏验证实验。在本研究中,我们首先证明了黄芩苷对 CORT 诱导的抑郁小鼠海马 NIX 介导的有丝分裂的影响。其次,我们在 HT22 细胞中分别转染 siRNA 敲除 AMPK、PGC-1α 和 NIX,并检测了黄芩苷对线粒体功能、AMPK/PGC-1α/NIX 通路蛋白表达和有丝分裂水平的影响。最后,向小鼠海马CA3注射AAV-shAMPKα以敲除AMPK,从而验证黄芩苷在体内的抗抑郁作用。结果表明,CORT诱导小鼠海马出现抑郁样行为,并伴有神经元损伤、线粒体损伤和抑制有丝分裂的作用,而黄芩苷(20 mg/kg)可以阻止这些行为。在 HT22 细胞中,黄芩苷能显著改善 CORT 诱导的线粒体功能障碍和有丝分裂障碍,而敲除 AMPK、PGC-1α 和 NIX 会阻断黄芩苷的保护作用。此外,在小鼠体内敲除 AMPKα 会抑制黄芩苷对抑郁样行为和 NIX 介导的有丝分裂的有益作用。我们的研究结果进一步证明,黄芩苷能促进 NIX 介导的有丝分裂,并以 AMPK 依赖性的方式改善抑郁症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


