Exosome-derived circ-001422 promotes tumor-associated macrophage M2 polarization to accelerate the progression of glioma
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
Cytokines, tumor cells, and tumor-associated macrophages play crucial roles in the composition of glioma tissue. Studies have demonstrated that certain cytokines can induce M2 polarization of tumor-associated macrophages and contribute to the progression of glioma. Nonetheless, the intricate molecular interactions among cytokines, glioma cells, and tumor-associated macrophages remain largely unexplored. To investigate this cross-talk, a combination of RNA-sequencing, chromatin immunoprecipitation, immunoprecipitation, exosome isolation, and biological experiments were employed. Treatment with IL-6 significantly increased circ-001422 expression in glioma cells. A poorer prognosis was associated with elevated levels of circ-001422 in glioma tissues. Circ-001422 was transcribed directly by STAT3 through binding to its promoter. Circ-001422 exerted cancer-promoting functions when co-cultured with M2 macrophages. Furthermore, glioma cells were found to transfer circ-001422 to macrophages via an exosomal pathway, promoting M2 polarization. Mechanically, circ-001422 interacted with p300, resulting in STAT3 acetylation, thus promoting nuclear localization and transcriptional activity of STAT3/NF-κB and M2 macrophage polarization. In conclusion, glioma cells released exosomes enriched with circ-001422, which in turn induce M2 macrophage polarization by activating the STAT3/NF-κB pathway, thereby enhancing the aggressive characteristics of glioma cells. Targeting circ-001422 may represent a potential therapeutic approach for glioma. Glioma cells release exosomes enriched with circ-001422, which in turn induce M2 macrophage polarization by activating the STAT3/NF-κB pathway, thereby enhancing the aggressive characteristics of glioma cells.
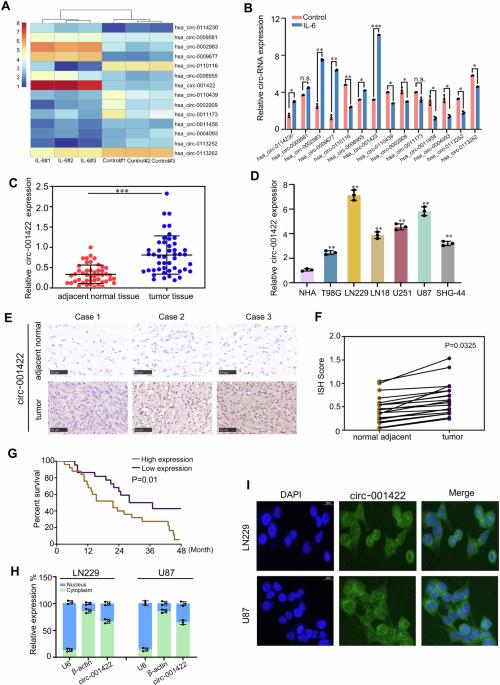
外泌体衍生的circ-001422可促进肿瘤相关巨噬细胞M2极化,从而加速胶质瘤的进展。
细胞因子、肿瘤细胞和肿瘤相关巨噬细胞在胶质瘤组织的构成中起着至关重要的作用。研究表明,某些细胞因子可诱导肿瘤相关巨噬细胞的 M2 极化,并导致胶质瘤的进展。然而,细胞因子、胶质瘤细胞和肿瘤相关巨噬细胞之间错综复杂的分子相互作用在很大程度上仍未得到探索。为了研究这种交叉作用,研究人员结合使用了RNA测序、染色质免疫沉淀、免疫沉淀、外泌体分离和生物实验。IL-6能明显增加胶质瘤细胞中circ-001422的表达。预后较差与胶质瘤组织中circ-001422水平升高有关。STAT3通过与其启动子结合直接转录circ-001422。当Circ-001422与M2巨噬细胞共同培养时,可发挥促癌功能。此外,研究还发现胶质瘤细胞可通过外泌体途径将circ-001422转移到巨噬细胞,从而促进M2极化。在机制上,circ-001422与p300相互作用,导致STAT3乙酰化,从而促进STAT3/NF-κB的核定位和转录活性,促进M2巨噬细胞极化。总之,胶质瘤细胞释放富含circ-001422的外泌体,进而通过激活STAT3/NF-κB通路诱导M2巨噬细胞极化,从而增强胶质瘤细胞的侵袭特性。以circ-001422为靶点可能是胶质瘤的一种潜在治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


