Neutrophil extracellular traps with low concentrations induce proliferation and migration of human fibroblasts via activating CCDC25/ILK/PI3K/AKT pathway
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-11-04
DOI:10.1016/j.bbrc.2024.150954
引用次数: 0
Abstract
Neutrophil plays an important role in the early stage of wound healing. Excessive neutrophil extracellular traps (NETs) which are produced by neutrophil have been proved as a significant unfavorable factor for wound healing by impairing the function of human skin fibroblasts. However, the effect of NETs with low concentrations on fibroblasts remains unclear. The interaction between CCDC25 and ILK in fibroblast were found to be activated by NET-DNA with low concentrations. Additionally, phosphorylation of PI3K and AKT was increased in NET-stimulated fibroblasts but was inhibited by CCDC25 knockout or ILK knockdown. NETs-induces proliferation and migration ability of fibroblast were also reduced by application of CCDC25 sgRNAs or ILK shRNAs. This study demonstrates that low concentrations of NETs, upon biding with CCDC25, activates the ILK/PI3K/AKT signaling pathway in skin fibroblasts, leading to increased cell proliferation and migration activity.
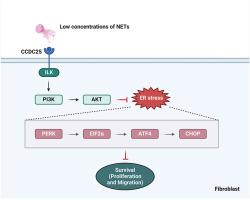
低浓度的中性粒细胞胞外捕获物通过激活 CCDC25/ILK/PI3K/AKT 通路诱导人成纤维细胞的增殖和迁移。
中性粒细胞在伤口愈合的早期阶段发挥着重要作用。由中性粒细胞产生的过多的中性粒细胞胞外捕获物(NETs)已被证实会损害人体皮肤成纤维细胞的功能,是影响伤口愈合的一个重要不利因素。然而,低浓度的NETs对成纤维细胞的影响仍不清楚。研究发现,低浓度的NET-DNA可激活成纤维细胞中CCDC25和ILK之间的相互作用。此外,在NET刺激的成纤维细胞中,PI3K和AKT的磷酸化增加,但CCDC25敲除或ILK敲除可抑制PI3K和AKT的磷酸化。应用 CCDC25 sgRNAs 或 ILK shRNAs 也会降低 NET 诱导的成纤维细胞增殖和迁移能力。这项研究表明,低浓度的NET与CCDC25结合后,会激活皮肤成纤维细胞中的ILK/PI3K/AKT信号通路,导致细胞增殖和迁移活性增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


