Potential mechanism of perillaldehyde in the treatment of nonalcoholic fatty liver disease based on network pharmacology and molecular docking
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
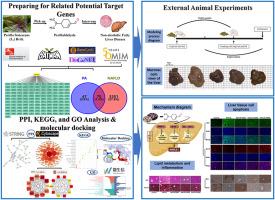
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic metabolic liver diseases worldwide. Perillaldehyde (4-propyl-1-en-2-ylcyclohexene-1-aldehyde, PA) is a terpenoid compound extracted from Perilla, which has effective pharmacological activities such as anti-inflammatory, antidepressant, and anticancer. This study aimed to explore the pharmacological effects of PA in intervening with NAFLD and reveal its potential mechanisms. Firstly, we identified the core targets of PA intervention therapy for NAFLD through network pharmacology and molecular docking techniques. After that, in vitro animal experiments such as H&E and Masson staining, immunofluorescence, immunohistochemistry, and Western blot were conducted to validate the results network effectively pharmacology predicted. Network pharmacology analysis suggested that PPAR-α may be the core target of PA intervention in NAFLD. H&E and Masson staining showed that after low-dose (50 mg/kg) PA administration, there was a noticeable improvement in fat deposition in the livers of NAFLD mice, and liver tissue fibrosis was alleviated. Immunohistochemical and immunofluorescence analysis showed that low dose (50 mg/kg) PA could reduce hepatocyte apoptosis, decrease the content of pro-apoptosis protein Bax, and increase the expression of anti-apoptosis protein Bcl-2 in NAFLD mice. Western blot results confirmed that low-dose (50 mg/kg) PA could increase the expression of PPAR-α and inhibit the expression of NF-κB in NAFLD mice. Our study indicated that PA could enhance the activity of PPAR-α and reduce the level of NF-κB in NAFLD mice, which may positively affect the prevention of NAFLD.
基于网络药理学和分子对接的紫苏醛治疗非酒精性脂肪肝的潜在机制
非酒精性脂肪肝(NAFLD)是全球最常见的慢性代谢性肝病之一。紫苏醛(4-丙基-1-烯-2-基环己烯-1-甲醛,PA)是从紫苏中提取的一种萜类化合物,具有抗炎、抗抑郁、抗癌等有效药理活性。本研究旨在探索PA干预非酒精性脂肪肝的药理作用,并揭示其潜在机制。首先,我们通过网络药理学和分子对接技术确定了 PA 干预治疗非酒精性脂肪肝的核心靶点。随后,通过H&E和Masson染色、免疫荧光、免疫组化和Western blot等体外动物实验验证了网络药理学预测的结果。网络药理学分析表明,PPAR-α可能是PA干预非酒精性脂肪肝的核心靶点。H&E和Masson染色显示,小剂量(50mg/kg)PA给药后,非酒精性脂肪肝小鼠肝脏脂肪沉积明显改善,肝组织纤维化减轻。免疫组化和免疫荧光分析表明,低剂量(50 毫克/千克)PA 可减少非酒精性脂肪肝小鼠肝细胞凋亡,降低促凋亡蛋白 Bax 的含量,增加抗凋亡蛋白 Bcl-2 的表达。Western印迹结果证实,低剂量(50 毫克/千克)PA 可增加非酒精性脂肪肝小鼠 PPAR-α 的表达,抑制 NF-κB 的表达。我们的研究表明,PA能增强非酒精性脂肪肝小鼠体内PPAR-α的活性,降低NF-κB的水平,这可能对预防非酒精性脂肪肝有积极作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


