Advancing osteosarcoma 3D modeling in vitro for novel tumor microenvironment-targeted therapies development
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Osteosarcoma (OS) represents one of the most common primary bone cancers affecting children and young adults. The available treatments have remained unimproved for the past decades, hampered by the poor knowledge of OS etiology/pathophysiology and the lack of innovative, predictive and biologically relevant in vitro models, that can recapitulate the 3D OS tumor microenvironment (TME). Here, we report the development and characterization of an innovative 3D model of OS, composed of OS tumor cells, immune cells (macrophages) and mesenchymal stem cells (MSCs), that formed a multicellular tissue spheroid (MCTS). This fully humanized 3D model was shown to accurately mimic the native histological features of OS, while innately leading to the polarization of macrophages towards an M2-like phenotype, highly aggressive and pro-tumor profile. Upon the exposure to immunomodulatory molecules, the MCTS were shown to be responsive by shifting macrophages polarization, and dramatically altering the TME secretome. In agreement, when treated with immunomodulatory/stimulatory nanoparticles (NPSs), we were able to revert the TME secretome towards an anti-inflammatory profile. This study establishes an advanced 3D OS model capable of shedding light on macrophages and MSCs contributions to disease progression, paving the way for the development of innovative therapeutic approaches targeting the OS TME, while providing a biologically relevant in vitro tool for the efficacy screening of novel OS therapeutic approaches.
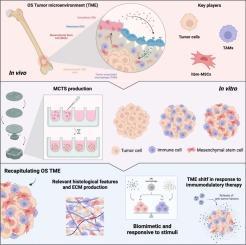
推进骨肉瘤三维体外建模,开发新型肿瘤微环境靶向疗法。
骨肉瘤(Osteosarcoma,OS)是儿童和年轻人最常见的原发性骨癌之一。由于对骨肉瘤的病因学/病理生理学知之甚少,以及缺乏可再现三维骨肉瘤肿瘤微环境(TME)的创新性、预测性和生物相关性体外模型,现有的治疗方法在过去几十年中一直没有得到改善。在此,我们报告了由 OS 肿瘤细胞、免疫细胞(巨噬细胞)和间充质干细胞(间充质干细胞)组成的 OS 创新三维模型的开发和特征描述,该模型形成了一个多细胞组织球体(MCTS)。研究表明,这种完全人源化的三维模型能准确模拟 OS 的原生组织学特征,同时导致巨噬细胞向 M2 样表型极化,具有高度侵袭性和促肿瘤特征。在暴露于免疫调节分子后,MCTS 通过改变巨噬细胞的极化和显著改变 TME 分泌组而显示出反应能力。同样,当使用免疫调节/刺激性纳米颗粒(NPSs)治疗时,我们能够将TME分泌组恢复到抗炎状态。这项研究建立了一种先进的三维 OS 模型,能够揭示巨噬细胞和间充质干细胞对疾病进展的影响,为开发针对 OS TME 的创新治疗方法铺平了道路,同时也为新型 OS 治疗方法的疗效筛选提供了一种生物相关的体外工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


