NMR coupled with multivariate data analysis for monitoring the degradation of a formulated therapeutic monoclonal antibody
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The function of a protein is directly coupled to its higher-order structure (HOS). Deviations in this critical quality attribute (CQA) may be linked to a decrease in the efficacy and/or safety of the final therapeutic product. High-resolution nuclear magnetic resonance (NMR) spectroscopy has been recently highlighted for protein HOS characterization, thanks to its ability to capture small changes at the molecular and structural levels (primary, secondary, tertiary and quaternary structures). The present study was carried out to demonstrate the ability of NMR (1D 1H and 2D 1H-13C experiments) coupled with multivariate data analysis (PCA and PLS regression) to monitor the degradation of a formulated mAb-like protein and for the simultaneous quantification of related CQAs, i.e., potency, purity and impurities (aggregates and fragments). The results indicate that this approach could be applied to mitigate product quality risks during development, manufacturing and stability studies of mAb therapeutics.
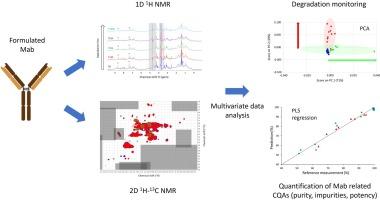
核磁共振与多元数据分析相结合,用于监测配制的治疗性单克隆抗体的降解。
蛋白质的功能与其高阶结构(HOS)直接相关。这一关键质量属性(CQA)的偏差可能会降低最终治疗产品的疗效和/或安全性。由于高分辨率核磁共振(NMR)光谱能够捕捉分子和结构层面(一级、二级、三级和四级)的微小变化,因此最近在蛋白质高阶结构表征方面备受关注。本研究展示了核磁共振(1D 1H 和 2D 1H-13C 实验)与多元数据分析(PCA 和 PLS 回归)相结合监测配方 mAb 类蛋白降解的能力,以及同时量化相关 CQAs(即效价、纯度和杂质(聚集体和片段))的能力。结果表明,这种方法可用于降低 mAb 疗法在开发、生产和稳定性研究过程中的产品质量风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


