Formulation optimization of chitosan surface coated solid lipid nanoparticles of griseofulvin: A Box-Behnken design and in vivo pharmacokinetic study
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
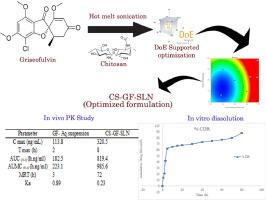
Solid lipid nanoparticles (SLNs) are becoming increasingly favored for their robust biocompatibility and their capacity to enhance drug solubility, particularly for drugs with limited water solubility. This study delves into the effectiveness of the hot melt sonication technique in fabricating SLNs with high drug loading capabilities and sustained release characteristics. Griseofulvin (GF), chosen as a representative drug due to its poor water solubility, was encapsulated into SLNs composed of stearic acid. Optimization of chitosan-coated GF-loaded SLNs (CS-GF-SLN) was conducted using a Box-Behnken design. Utilizing the desirability approach, optimal parameters were determined, including a lipid quantity of 450.593 mg, chitosan content of 268.67 mg, and sonication duration of 2.14 h. These parameters resulted in a zeta potential of -34.8 mV and a particle size (PS) of 56.87 nm. Following optimization, the refined formulation underwent comprehensive assessment across various parameters. Notably, the drug encapsulated within SLNs exhibited sustained release over three days, as illustrated by the in-vitro drug release profile. The optimized formulation demonstrated a bioavailability enhancement by approximately 1.7 to 2.0 times compared to the conventional formulation. Furthermore, administration of drug-loaded SLNs to a macrophage cell line demonstrated no cytotoxicity, affirming their suitability as conventional drug delivery platforms for GF.
壳聚糖表面包覆格列齐芬固体脂质纳米颗粒的配方优化:Box-Behnken 设计和体内药代动力学研究
固体脂质纳米颗粒(SLNs)因其强大的生物相容性和提高药物溶解度的能力而日益受到青睐,尤其是对水溶性有限的药物而言。本研究探讨了热熔超声技术在制造具有高药物负载能力和持续释放特性的 SLNs 方面的有效性。Griseofulvin (GF) 因其水溶性较差而被选为代表性药物,该药物被封装在由硬脂酸组成的 SLNs 中。采用盒-贝肯设计法对壳聚糖包覆的 GF 负载 SLN(CS-GF-SLN)进行了优化。利用可取性方法确定了最佳参数,包括脂质量 450.593 毫克、壳聚糖含量 268.67 毫克和超声持续时间 2.14 小时。通过这些参数,zeta 电位为 -34.8 mV,粒度(PS)为 56.87 nm。优化后,对改进后的配方进行了各种参数的综合评估。值得注意的是,正如体外药物释放曲线所示,包裹在 SLNs 中的药物在三天内呈现持续释放状态。与传统制剂相比,优化制剂的生物利用度提高了约 1.7 至 2.0 倍。此外,对巨噬细胞系施用药物负载的 SLNs 没有显示出细胞毒性,这肯定了它们作为 GF 传统药物递送平台的适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


