Novel förster resonance energy transfer (FRET)-based ratiometric fluorescent probe for detection of cyanides by nucleophilic substitution of aromatic hydrogen (SNArH)
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-10-28
DOI:10.1016/j.saa.2024.125339
引用次数: 0
Abstract
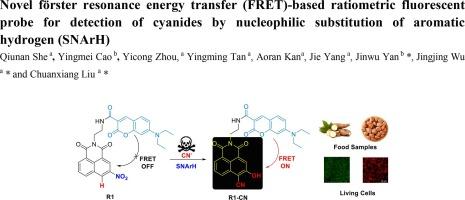
The development of novel fluorescent probes for real-time detection of cyanides (CN−) in environmental and biological systems has become a significant focus in chemical sensing. Particularly, ratiometric fluorescence sensing offers a unique method for precise and quantitative detection of cyanides, even under complex conditions. We report herein the design of a new ratiometric fluorescent probe for cyanides based on modulation of Förster resonance energy transfer (FRET) coupled with novel cyanide-induced nucleophilic substitution of aromatic hydrogen (SNArH). The target probe (R1) is developed by introducing coumarin fluorophores as FRET donors into a 3-nitro-naphthalimide acceptor, which is easily synthesized and exhibits a colorimetric change from colorless to faint yellow and a significant ratiometric fluorescence shift (Δλ = 114 nm) upon cyanide binding. A clear ratiometric signal at I582/I468 was obtained, with a limit of detection of 5.69 μM. The sensing mechanism was confirmed through 1H NMR titration and LC-MS analysis. Additionally, R1-loaded strips were easily prepared, serving as a portable device for detecting CN− with visible color changes. The probe R1 has been successfully utilized for real-time monitoring of cyanide in food materials and water samples. Importantly, fluorescence bioimaging studies in HeLa cells were conducted, demonstrating the probe’s capability for ratiometric detection of exogenous CN− in living systems.
基于费斯特共振能量转移(FRET)的新型比率荧光探针,用于通过亲核取代芳香族氢(SNArH)检测氰化物
开发用于实时检测环境和生物系统中氰化物(CN-)的新型荧光探针已成为化学传感领域的一个重要焦点。尤其是比率荧光传感技术,它提供了一种即使在复杂条件下也能精确定量检测氰化物的独特方法。我们在此报告了一种新的氰化物比率荧光探针的设计,该探针基于福斯特共振能量转移(FRET)调制和新型氰化物诱导的芳香氢亲核取代(SNArH)。目标探针(R1)是通过在 3-硝基萘二甲酰亚胺受体中引入香豆素荧光团作为 FRET 给体而开发的,该受体易于合成,在与氰化物结合后,其色度会从无色变为淡黄色,并出现明显的比率荧光偏移(Δλ = 114 nm)。在 I582/I468 处获得了清晰的比率信号,检测限为 5.69 μM。感应机制通过 1H NMR 滴定和 LC-MS 分析得到了证实。此外,加载 R1 的试剂条也很容易制备,可作为一种便携式装置,通过可见的颜色变化来检测 CN-。探针 R1 已成功用于食品材料和水样中氰化物的实时监测。重要的是,还在 HeLa 细胞中进行了荧光生物成像研究,证明该探针能够在活体系统中按比率检测外源 CN-。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


