Conquering dual challenges: A sialic-modified liposome for targeting activated neutrophils to tackle comorbid lung inflammation and cancer metastasis
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In clinical settings, cancer frequently coexists with multi-system diseases. Owing to compromised immune systems, patients with cancer exhibit an increased susceptibility to infections and inflammation. Notably, lung inflammation occurs with high incidence among these patients. Furthermore, the inflammatory milieu within the lungs often accelerates the metastasis of cancer, thereby enhancing mortality rates and posing substantial challenges for clinical management. To date, effective strategies addressing both lung inflammation and cancer concurrently are lacking. In this context, we introduce a novel therapeutic approach involving a sialic acid-lipid derivative (SA-PG10-C18) modified doxorubicin-curcumin co-loaded liposome (DOX/CUR-SAL). This formulation effectively targeted activated neutrophils, which are abundantly present in inflammatory and metastatic lung tissues. DOX/CUR-SAL notably inhibited neutrophil-mediated pro-inflammatory and pro-metastatic processes. Utilizing a newly established mouse model of acute lung injury (ALI) and metastasis comorbidity, DOX/CUR-SAL modulated the lung immune microenvironment and arrested the progression of both inflammation and metastasis, without inducing side effects. The treated animals demonstrated favorable survival conditions, persisting beyond 45 days. This innovative therapeutic strategy offers a novel concept and reference for treating comorbid conditions of tumors and inflammation, thus breaking the clinical impasse where lung inflammation and cancer metastasis have been treated separately.
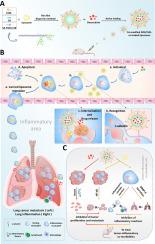
征服双重挑战:以活化的中性粒细胞为靶标,解决肺部炎症和癌症转移并发症的硅胶修饰脂质体。
在临床上,癌症经常与多系统疾病并存。由于免疫系统受损,癌症患者对感染和炎症的易感性增加。值得注意的是,肺部炎症在这些患者中发病率很高。此外,肺部的炎症环境往往会加速癌症的转移,从而提高死亡率,给临床治疗带来巨大挑战。迄今为止,还缺乏同时应对肺部炎症和癌症的有效策略。在此背景下,我们介绍了一种新的治疗方法,它涉及一种经硅酸-脂质衍生物(SA-PG10-C18)修饰的多柔比星-姜黄素共载脂质体(DOX/CUR-SAL)。这种制剂能有效靶向活化的中性粒细胞,而中性粒细胞大量存在于炎症和转移性肺组织中。DOX/CUR-SAL 能显著抑制中性粒细胞介导的促炎症和促转移过程。利用新建立的急性肺损伤(ALI)和转移合并症小鼠模型,DOX/CUR-SAL 调节了肺部免疫微环境,阻止了炎症和转移的发展,且不会产生副作用。接受治疗的动物显示出良好的存活条件,存活时间超过 45 天。这种创新的治疗策略为治疗肿瘤和炎症并发症提供了新的理念和参考,从而打破了肺部炎症和癌症转移分别治疗的临床僵局。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


