Understanding the role of CaCl2 in salt substitute for low-salt and high-quality surimi products
IF 6.2
2区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Salt substitute has been widely used to prepare low-salt foods due to potential health benefits, though the role of CaCl2 in salt substitute and its unique impacts on food quality have been rarely investigated. In this study, comprehensive research has been conducted to elucidate the effects of replacing NaCl with varying concentrations of CaCl2 on the surimi gel characteristics. The introduction of CaCl2 interacted with surimi proteins differently from NaCl, thus leading to difference in protein aggregation behaviors and surimi gel properties. It has been found that a proper proportion of CaCl2 for NaCl substitution could create salt bridges between surimi proteins more effectively, resulting in an ordered, smooth and dense gel network with an increased water holding capacity (WHC) and improved gel strength. Furthermore, TGase activated by Ca2+ boosted the formation of ε-(γ-glutamyl) lysine bonds, which cross-linked surimi proteins to form a firm gel with a better three-dimensional structure. However, replacing NaCl with excessive amount of CaCl2 as divalent salts induced more serious protein aggregation, leading to water loss and gel properties deterioration. More specially, replacing NaCl with CaCl2 at 50% showed the best performance, as evidenced by the most abundant disulfide bonds and hydrophobic interactions, highest hardness and chewiness, and greatest storage modulus. This study provided new insights on developing high-quality surimi gels with significantly reduced salt concentration and improved gel characteristics.
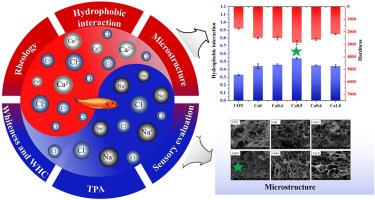
了解 CaCl2 在低盐优质鱼糜产品代盐中的作用
由于潜在的健康益处,代盐已被广泛用于制备低盐食品,但人们很少研究 CaCl2 在代盐中的作用及其对食品质量的独特影响。本研究进行了全面研究,以阐明用不同浓度的 CaCl2 替代 NaCl 对鱼糜凝胶特性的影响。引入的 CaCl2 与鱼糜蛋白质的相互作用与 NaCl 不同,从而导致蛋白质聚集行为和鱼糜凝胶特性的差异。研究发现,用适当比例的 CaCl2 替代 NaCl 能更有效地在鱼糜蛋白质之间建立盐桥,从而形成有序、光滑和致密的凝胶网络,并提高持水量(WHC)和凝胶强度。此外,Ca2+激活的TG酶还能促进ε-(γ-谷氨酰)赖氨酸键的形成,从而交联鱼糜蛋白,形成具有更好三维结构的坚固凝胶。然而,用过多的二价盐 CaCl2 取代 NaCl 会诱发更严重的蛋白质聚集,导致水分流失和凝胶性能下降。更特别的是,用 50%的 CaCl2 取代 NaCl 的性能最佳,表现在二硫键和疏水作用最丰富、硬度和咀嚼性最高、储存模量最大。这项研究为开发盐浓度显著降低、凝胶特性得到改善的优质鱼糜凝胶提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Food Science
Agricultural and Biological Sciences-Food Science
CiteScore
7.40
自引率
3.20%
发文量
232
审稿时长
84 days
期刊介绍:
Current Research in Food Science is an international peer-reviewed journal dedicated to advancing the breadth of knowledge in the field of food science. It serves as a platform for publishing original research articles and short communications that encompass a wide array of topics, including food chemistry, physics, microbiology, nutrition, nutraceuticals, process and package engineering, materials science, food sustainability, and food security. By covering these diverse areas, the journal aims to provide a comprehensive source of the latest scientific findings and technological advancements that are shaping the future of the food industry. The journal's scope is designed to address the multidisciplinary nature of food science, reflecting its commitment to promoting innovation and ensuring the safety and quality of the food supply.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


