Comparative analysis of purity of human albumin preparations for clinical use
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
Albumin is the most prevalent plasma protein and serves numerous physiological roles, both in body fluid management and in various other capacities. In many diseases, a deficiency of albumin has been observed, and in certain conditions, albumin substitution has been demonstrated to improve outcome in comparison to plasma expansion using crystalloid or other colloid solutions. The favourable effects of using albumin in patients with liver cirrhosis are likely associated with the non-oncotic functions of albumin. Albumin for clinical use is obtained through fractionation of pooled donor plasma. The production procedures are optimized to ensure pure, chemically uncompromised and native protein.
Results
We have extensively analysed commercial preparations of human albumin for clinical use from six different providers. Parameters that must correspond to the requirements of international pharmacopoeias were assessed (aluminium, ethanol, sodium, the presence of dimers and oligomers) and found to conform in all cases. In addition, we used for the first time nuclear magnetic resonance (NMR) as an additional analytical approach for investigating in greater depth the quality of a biological remedy gained from human plasma. We applied both 1H NMR and 13C-HSQC for confirming the identity of the albumin preparations, which also conformed in all cases. Moreover, we utilized T2-filtered 1H NMR and 13C-HSQC measurements to identify the presence of small molecules in the preparations. This demonstrated similar patterns of additional substances present, but also unveiled certain differences in purity in the products of the different providers.
Significance
Our analyses confirmed that albumin preparations in clinical use conform to the requirements. We furthermore demonstrate that NMR measurements can provide further depth in identity and purity measurements of biologicals. Despite largely standardized protocols in pharmaceutical albumin production, our in-depth analyses revealed differences in purity. Some samples exhibited lower levels of components other than albumin. We discuss possible causes of these observations and their potential implications for clinical therapy.
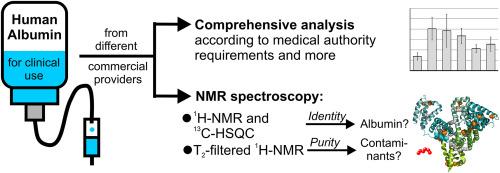
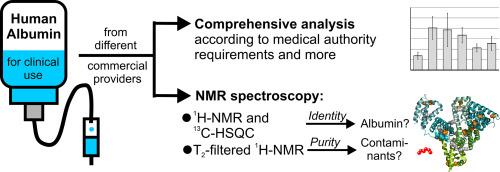
临床用人血白蛋白制剂纯度对比分析
背景白蛋白是最常见的血浆蛋白,在体液管理和其他各种功能中发挥着多种生理作用。在许多疾病中都可观察到白蛋白缺乏,在某些情况下,白蛋白替代品与使用晶体液或其他胶体溶液进行血浆扩容相比,可改善预后。肝硬化患者使用白蛋白的良好效果可能与白蛋白的非抗凝血功能有关。临床使用的白蛋白是通过对集合供体血浆进行分馏获得的。我们广泛分析了六家不同供应商提供的临床用人血白蛋白商业制剂。对必须符合国际药典要求的参数(铝、乙醇、钠、二聚体和低聚物的存在)进行了评估,发现所有参数都符合要求。此外,我们还首次将核磁共振(NMR)作为一种额外的分析方法,用于更深入地研究从人体血浆中提取的生物药剂的质量。我们同时使用 1H-NMR 和 13C-HSQC 来确认白蛋白制剂的身份,结果也全部吻合。此外,我们还利用 T2 过滤 1H-NMR 和 13C-HSQC 测量来确定制剂中是否存在小分子物质。我们的分析证实,临床使用的白蛋白制剂符合要求。我们的分析证实了临床使用的白蛋白制剂符合要求,并进一步证明了 NMR 测量可进一步深入生物制品的特性和纯度测量。尽管药用白蛋白的生产规程基本标准化,但我们的深入分析发现了纯度方面的差异。一些样品中除白蛋白以外的其他成分含量较低。我们讨论了这些观察结果的可能原因及其对临床治疗的潜在影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


