Plasma-driven non-oxidative coupling of methane to ethylene and hydrogen at mild temperature over CuxO/CeO2 catalyst
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We report one-step non-oxidative coupling of methane (CH4) to ethylene (C2H4) at atmospheric pressure and mild temperature (ca. 180–190 °C), by a combination of non-thermal plasma and a CuOx/CeO2 catalyst. The C2H4 selectivity gradually increases during an induction period. The corresponding spent catalysts at different stages were systematically characterized to disclose the evolution of the CuOx/CeO2 catalyst. During the induction period, the CuO/CeO2 catalyst was partially reduced to generate Cu+, Ce3+ and Ov species, which accompany the formation of Cu+-Ov-Ce3+ sites, as proven by XRD, HRTEM, XPS, Raman, EPR and H2-TPR. In addition, the C2H4 selectivity is proportional to the fraction of Cu+, Ce3+, Ov and Cu-O-Ce species, which indicates that Cu+-Ov-Ce3+ is the active site for non-oxidative coupling of CH4 to C2H4. Furthermore, in-situ FTIR results indicate that the Cu+-Ov-Ce3+ interface sites can promote dehydrogenation of CH3* (from CH4 plasma) to produce CH2* on the catalyst surface, which is the basic reason why CuOx/CeO2 acts as a catalyst in speeding up the non-oxidative coupling of CH4 for C2H4 production.
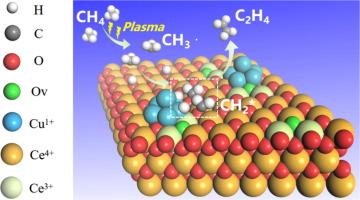
在 CuxO/CeO2 催化剂上低温等离子体驱动甲烷与乙烯和氢的非氧化偶联反应
我们报告了在常压和温和温度(约 180-190 °C)下,通过非热等离子体和 CuOx/CeO2 催化剂的组合,一步法非氧化偶联甲烷(CH4)和乙烯(C2H4)的情况。在诱导期,C2H4 的选择性逐渐增加。对不同阶段的相应废催化剂进行了系统表征,以揭示 CuOx/CeO2 催化剂的演变过程。通过 XRD、HRTEM、XPS、拉曼、EPR 和 H2-TPR 可以证明,在诱导期间,CuO/CeO2 催化剂被部分还原,生成 Cu+、Ce3+ 和 Ov 物种,伴随着 Cu+-Ov-Ce3+ 位点的形成。此外,C2H4 的选择性与 Cu+、Ce3+、Ov 和 Cu-O-Ce 物种的比例成正比,这表明 Cu+-Ov-Ce3+ 是 CH4 与 C2H4 非氧化偶联的活性位点。此外,原位傅立叶变换红外光谱结果表明,Cu+-Ov-Ce3+界面位点可促进催化剂表面的 CH3*(来自 CH4 等离子体)脱氢生成 CH2*,这是 CuOx/CeO2 作为催化剂加速 CH4 非氧化偶联生成 C2H4 的根本原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


