Scale-Up Preparation of Best-In-Class Orally Bioavailable CXCR4 Antagonist EMU-116 in an Academic Setting
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
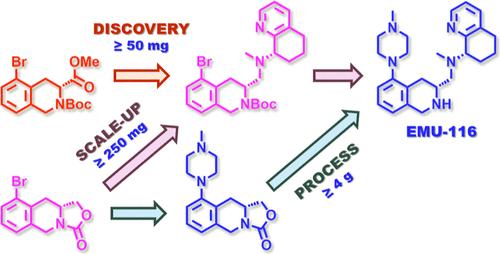
CXCR4 is a seven-transmembrane chemokine receptor that is intimately involved in stem cell niche maintenance and immune cell trafficking. Among several other pathophysiological states for which CXCR4 mis regulation is implicated, various hematological malignancies and solid tumors hijack this chemokine network by dramatically overexpressing CXCR4 and its cognate chemokine ligand CXCL12. Upregulation of the CXCR4/CXCL12 axis in cancer drives tumor progression through several mechanisms, which makes CXCR4 a promising target for the development of anticancer therapeutics. Herein, we report the preparative scale synthesis of a novel, best-in-class, orally bioavailable small molecule CXCR4 antagonist, EMU-116. Two synthetic strategies for production of EMU-116 were pursued. While the first discovery-focused synthesis facilitated late-stage diversification to drive structure–activity relationship determinations, the second process-focused synthesis delivered EMU-116 more efficiently in higher overall yield with enhanced stereocontrol. For both synthetic routes, Buchwald–Hartwig amination of key aryl bromide intermediates enabled installation of the N-methylpiperazine appendage of EMU-116. Synthetic methods devised to prepare (R)-9-bromo-1,5,10,10a-tetrahydro-3H-oxazolo[3,4-b]isoquinolin-3-one, the key aryl bromide intermediate required for the process-focused synthesis, are reported. In addition, an improved preparative method of known synthon (S)–N-methyl-5,6,7,8-tetrahydroquinolin-8-amine is highlighted by elevated overall yield, enhanced diastereoselectivity, and robust purification by crystallization. Further elaboration of these two intermediates, coupling via reductive amination to furnish the full EMU-116 scaffold, removal of protecting groups, and final product purification techniques are also reported. Overall, the synthetic methods described herein enabled reliable and efficient production of multigram quantities of EMU-116 and are anticipated to be amenable to larger scale production.
在学术环境中扩大同类最佳口服生物可用性 CXCR4 拮抗剂 EMU-116 的制备规模
CXCR4 是一种七跨膜趋化因子受体,与干细胞龛维持和免疫细胞贩运密切相关。在其他几种与 CXCR4 调控失误有关的病理生理状态中,各种血液恶性肿瘤和实体瘤通过显著过表达 CXCR4 及其同源趋化因子配体 CXCL12 来劫持这一趋化因子网络。癌症中 CXCR4/CXCL12 轴的上调通过多种机制推动肿瘤进展,这使得 CXCR4 成为开发抗癌疗法的一个有前景的靶点。在此,我们报告了一种新型、同类最佳、口服生物可用性小分子 CXCR4 拮抗剂 EMU-116 的制备规模合成。我们采用了两种合成策略来生产 EMU-116。第一种以发现为中心的合成有助于后期的多样化,以推动结构-活性关系的确定,而第二种以工艺为中心的合成则以更高的总产率和更强的立体控制更有效地生产出 EMU-116。在这两种合成路线中,关键芳基溴化物中间体的布赫瓦尔德-哈特维格胺化反应使 EMU-116 的 N-甲基哌嗪附属物得以安装。报告中介绍了制备 (R)-9-bromo-1,5,10,10a-tetrahydro-3H-oxazolo[3,4-b]isoquinolin-3-one 的合成方法,该方法是以工艺为重点的合成所需的关键芳基溴化中间体。此外,通过提高总收率、非对映选择性和结晶纯化能力,重点介绍了已知合成物 (S)-N- 甲基-5,6,7,8-四氢喹啉-8-胺的改进制备方法。报告还介绍了这两种中间体的进一步阐述、通过还原胺化耦合以提供完整的 EMU-116 支架、去除保护基团以及最终产品纯化技术。总之,本文所述的合成方法能够可靠、高效地生产多克量的 EMU-116,预计可用于更大规模的生产。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


